
What is $P{H_3}$?
Answer
503.4k+ views
Hint: Phosphine or hydrogen phosphide is a flammable, colourless, extremely toxic gas having a disagreeable garlic-like odour. Moreover, phosphine is classed as pictogen hydride. Phosphine is usually used in semiconductor or plastic industries and even in the production of flame retardants.
Complete answer:
Phosphine is the compound with the chemical formula $P{H_3}$.
The IUPAC name for $P{H_3}$ is phosphine. Phosphine is also known as hydrogen phosphide.
Phosphine was first obtained in 1783 by Philippe Gengembre, by heating white phosphorus in an aqueous solution of potassium carbonate.
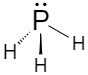
Phosphine has a trigonal pyramidal structure. Length of the P-H bond is $1.42{A^{^\circ }}$ and the H-P-H bond angle is ${93.5^ \circ }$.
Around the phosphorus atom, there are three sigma bonds and one lone pair.
Explanation of not well defined or absence of $P{H_3}$ hybridization can be given by Drago’s rule. During the formation of phosphine, the pure p orbitals participate in bonding and avoid getting hybridized.
Phosphine burns to produce a dense white cloud of phosphoric acid.
$P{H_3} + 2{O_2} \to {H_3}P{O_4}$
Phosphine dissolves less readily in water but more readily in non-polar solvents.
Phosphine is a Lewis base because of the presence of a nonbonding electron pair on the p- shell that can be donated.
Phosphine can be prepared in many ways. Industrially, it is prepared by the reaction of white phosphorus with potassium hydroxide, producing potassium hypophosphite as a by-product.
$3KOH + {P_4} + 3{H_2}O \to 3K{H_2}P{O_2} + P{H_3}$
And in the laboratory it can be prepared by disproportionation of phosphoric acid.
$4{H_3}P{O_3} \to P{H_3} + 3{H_3}P{O_4}$
Note:
Phosphine has an unpleasant odour like rotting fish and it is a highly toxic respiratory poison. Phosphine is mainly a redox toxin, which can cause cell damage by inducing oxidative stress and mitochondrial dysfunction, while it produces resistance in pests by a mutation in the mitochondrial metabolic gene.
Complete answer:
Phosphine is the compound with the chemical formula $P{H_3}$.
The IUPAC name for $P{H_3}$ is phosphine. Phosphine is also known as hydrogen phosphide.
Phosphine was first obtained in 1783 by Philippe Gengembre, by heating white phosphorus in an aqueous solution of potassium carbonate.
Phosphine has a trigonal pyramidal structure. Length of the P-H bond is $1.42{A^{^\circ }}$ and the H-P-H bond angle is ${93.5^ \circ }$.
Around the phosphorus atom, there are three sigma bonds and one lone pair.
Explanation of not well defined or absence of $P{H_3}$ hybridization can be given by Drago’s rule. During the formation of phosphine, the pure p orbitals participate in bonding and avoid getting hybridized.

Phosphine burns to produce a dense white cloud of phosphoric acid.
$P{H_3} + 2{O_2} \to {H_3}P{O_4}$
Phosphine dissolves less readily in water but more readily in non-polar solvents.
Phosphine is a Lewis base because of the presence of a nonbonding electron pair on the p- shell that can be donated.
Phosphine can be prepared in many ways. Industrially, it is prepared by the reaction of white phosphorus with potassium hydroxide, producing potassium hypophosphite as a by-product.
$3KOH + {P_4} + 3{H_2}O \to 3K{H_2}P{O_2} + P{H_3}$
And in the laboratory it can be prepared by disproportionation of phosphoric acid.
$4{H_3}P{O_3} \to P{H_3} + 3{H_3}P{O_4}$
Note:
Phosphine has an unpleasant odour like rotting fish and it is a highly toxic respiratory poison. Phosphine is mainly a redox toxin, which can cause cell damage by inducing oxidative stress and mitochondrial dysfunction, while it produces resistance in pests by a mutation in the mitochondrial metabolic gene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




