
What is Hinsberg reagent?
Answer
570k+ views
Hint: An attempt to this question can be made by determining the composition of Hinsberg reagent. It is important to know that the reagent is used to detect the degree of amine present in the solution. Based on the reaction mechanism determine the structure of compound present in Hinsberg reagent and thus identify the name of the compound in accordance with IUPAC nomenclature.
Complete answer:
Hinsberg reagent is an alternate name given for the compound, benzene sulfonyl chloride. This name is given for its role in the Hinsberg test.
The Hinsberg test is mainly used for the detection and distinction of primary, secondary and tertiary amines in a given solution.
The reagent comes under the class of organosulfur compounds in the field of organic chemistry.
The structure of the compound is given below:
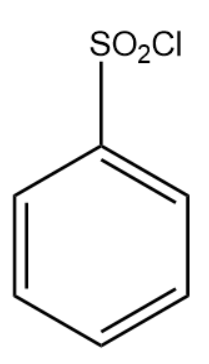
Hinsberg reagent is a colorless oil type of substance. It is viscous in nature and soluble in organic solvents.
This reaction undergoes reactions with organic compounds that contain O - H and N - H bonds that are considered to be reactive in nature.
It is used for the preparation of sulfonamides and sulfonamide esters. Sulfonamides are formed by the reaction of reagents with amines and sulfonamide esters with alcohols.
Note:
It is important to know the reason why the Hinsberg reagent is soluble in only organic solvents. Benzene sulfonyl chloride does not contain replaceable protons. Thus, it cannot form bonds with polar solvents like water. On the other hand, in aprotic solvents, it can dissolve easily without the supply of energy.
Complete answer:
Hinsberg reagent is an alternate name given for the compound, benzene sulfonyl chloride. This name is given for its role in the Hinsberg test.
The Hinsberg test is mainly used for the detection and distinction of primary, secondary and tertiary amines in a given solution.
The reagent comes under the class of organosulfur compounds in the field of organic chemistry.
The structure of the compound is given below:

Hinsberg reagent is a colorless oil type of substance. It is viscous in nature and soluble in organic solvents.
This reaction undergoes reactions with organic compounds that contain O - H and N - H bonds that are considered to be reactive in nature.
It is used for the preparation of sulfonamides and sulfonamide esters. Sulfonamides are formed by the reaction of reagents with amines and sulfonamide esters with alcohols.
Note:
It is important to know the reason why the Hinsberg reagent is soluble in only organic solvents. Benzene sulfonyl chloride does not contain replaceable protons. Thus, it cannot form bonds with polar solvents like water. On the other hand, in aprotic solvents, it can dissolve easily without the supply of energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




