
What is distillation?
Answer
581.7k+ views
Hint: A lot of techniques are there to separate mixtures of compounds into individual compounds. Out of those techniques distillation is one of the methods to separate the mixture of two different liquid components.
Complete Solution :
- Distillation is a method to separate a mixture of liquid components into individual liquid components.
- The separation of liquids is based on the boiling points of the liquid components.
- Distillation is not a chemical reaction.
- Distillation process is a physical phenomenon because we are going to separate the two or more different liquids physically from one another based on their boiling point.
- First we will take the mixture of liquid components in a round bottom flask.
- Later we are going to start heating the liquid mixture.
- After a certain time the liquid mixture is going to boil, at this time the liquid component which has a low boiling point comes out first and the remaining liquid components come out basing on their boiling points.
- The liquid components which are coming out the distillation flask are going to be condensed by using a condenser and we can see it in the below picture.
- The setup for the distillation process is as follows :
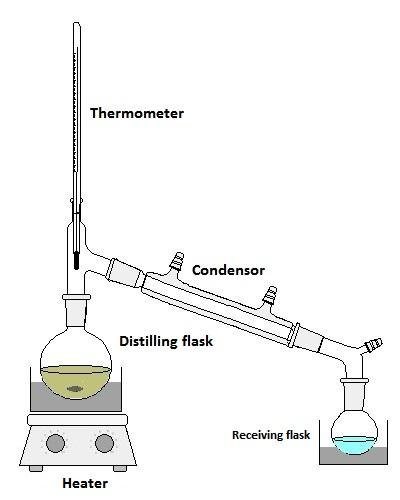
- If the liquid components have the same boiling points, we can separate them by using a fractional distillation process.
Note: There are different types of distillation processes available. These different types of distillation process are going to be used on the bases of boiling points and the presence of different impurities in the liquid mixtures.
Complete Solution :
- Distillation is a method to separate a mixture of liquid components into individual liquid components.
- The separation of liquids is based on the boiling points of the liquid components.
- Distillation is not a chemical reaction.
- Distillation process is a physical phenomenon because we are going to separate the two or more different liquids physically from one another based on their boiling point.
- First we will take the mixture of liquid components in a round bottom flask.
- Later we are going to start heating the liquid mixture.
- After a certain time the liquid mixture is going to boil, at this time the liquid component which has a low boiling point comes out first and the remaining liquid components come out basing on their boiling points.
- The liquid components which are coming out the distillation flask are going to be condensed by using a condenser and we can see it in the below picture.
- The setup for the distillation process is as follows :

- If the liquid components have the same boiling points, we can separate them by using a fractional distillation process.
Note: There are different types of distillation processes available. These different types of distillation process are going to be used on the bases of boiling points and the presence of different impurities in the liquid mixtures.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Trending doubts
Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Difference Between Plant Cell and Animal Cell

What is the color of ferrous sulphate crystals? How does this color change after heating? Name the products formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this type of change.

Find the greatest fivedigit number which is a perfect class 9 maths CBSE

Find the mode and median of the data 13 16 12 14 1-class-9-maths-CBSE

What is pollution? How many types of pollution? Define it




