
What is covalency?
Answer
596.1k+ views
Hint: A covalent bond is formed between two atoms by sharing of electrons. The chemical activity and reactivity of an atom is determined by the number of electrons present in its outermost shell.
Complete step by step solution:
The number of electrons in the outermost shell of an atom is known as its valency. If the number of electrons in the outermost shell of an atom is less than the total electrons in its fully filled configuration, then the atom has a tendency to reach the nearest noble gas configuration. It may do so either by sharing its electrons with other atoms or by losing and gaining electrons.
When an atom attains the stable configurations by losing or gaining electrons (or electrons), the number of electrons lost or gained is called its electrovalency. For e.g. In NaCl, Na has one electron is its outermost shell (\[Na:1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}\]). It loses that one electron to attain stable noble gas configuration of Ne.
When an atom shares its electrons with electrons of other atoms (or atom), the number of electrons shared by the atom is called its covalency. The compounds formed by sharing of electrons between atoms are called covalent compounds. Covalency is also defined as the maximum number of bonds formed by an atom to reach the stable electronic configuration.
Carbon has four electrons in its valence or outermost shell. It requires four more electrons to fill up the shell. In methane (\[C{{H}_{4}}\]) four hydrogen atoms, each having one electron, share their electrons with four electrons of carbon. Four covalent \[C-H\] bonds are formed completing the electronic configurations of both hydrogen and carbon.
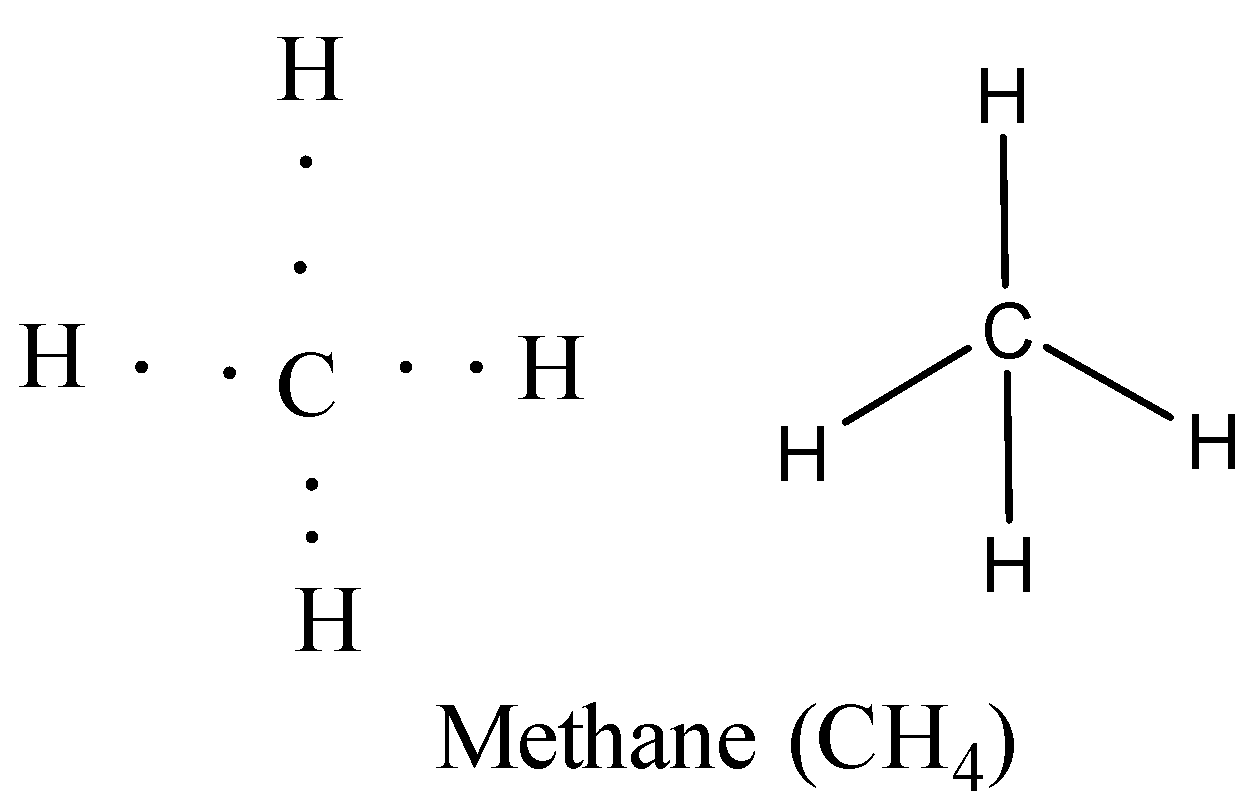 Here, the number of bonds formed by carbon is four. Hence, the covalency of carbon is 4.
Here, the number of bonds formed by carbon is four. Hence, the covalency of carbon is 4.
Additional Information: Covalency is maximum when the electrons are shared between the atoms of comparable electronegativities. If the number of electrons present in the outermost shell of an atom is more than 4, then its valency is equal to 8 minus the number of electrons present.
Note: Do not confuse valency with covalency. Valency is the total number of electrons available for bonding (for loosing, gaining or sharing) whereas covalency is the number of bonds formed by an atom, each bond equals two electrons.
Complete step by step solution:
The number of electrons in the outermost shell of an atom is known as its valency. If the number of electrons in the outermost shell of an atom is less than the total electrons in its fully filled configuration, then the atom has a tendency to reach the nearest noble gas configuration. It may do so either by sharing its electrons with other atoms or by losing and gaining electrons.
When an atom attains the stable configurations by losing or gaining electrons (or electrons), the number of electrons lost or gained is called its electrovalency. For e.g. In NaCl, Na has one electron is its outermost shell (\[Na:1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}\]). It loses that one electron to attain stable noble gas configuration of Ne.
When an atom shares its electrons with electrons of other atoms (or atom), the number of electrons shared by the atom is called its covalency. The compounds formed by sharing of electrons between atoms are called covalent compounds. Covalency is also defined as the maximum number of bonds formed by an atom to reach the stable electronic configuration.
Carbon has four electrons in its valence or outermost shell. It requires four more electrons to fill up the shell. In methane (\[C{{H}_{4}}\]) four hydrogen atoms, each having one electron, share their electrons with four electrons of carbon. Four covalent \[C-H\] bonds are formed completing the electronic configurations of both hydrogen and carbon.

Additional Information: Covalency is maximum when the electrons are shared between the atoms of comparable electronegativities. If the number of electrons present in the outermost shell of an atom is more than 4, then its valency is equal to 8 minus the number of electrons present.
Note: Do not confuse valency with covalency. Valency is the total number of electrons available for bonding (for loosing, gaining or sharing) whereas covalency is the number of bonds formed by an atom, each bond equals two electrons.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




