
What is a silicon polymer?
Answer
540.3k+ views
Hint: Polymer means there is a repeating unit called monomer which is repeated numerous times to form a macromolecule and in silicon polymer, their repeating unit must contain silicon atoms.
Complete step-by-step answer:Polymer means there is a repeating unit called monomer which is repeated numerous times to form a macromolecule. Mostly the polymers are carbon-containing monomers, but there is another class of polymers called silicon polymer or polysiloxane. In these, the backbone chain is not formed by carbon atoms, it is made from silicon and oxygen atoms. Its monomer unit is called siloxane and it is represented as $(-{{R}_{2}}Si-O-Si{{R}_{2}}-)$, in which R is the alkyl group. The basic structure of silicon polymer is given below:
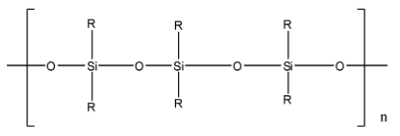
These polymers have many good and useful properties like they are water repellent, have heat stability, they have good resistance to chemical attack. These are used in many varieties like rubber-material, oils, grease, adhesives, medicines, cooking utensils, etc.
We can prepare a siloxane, polydimethylsiloxane by the hydrolysis of dimethyldichlorosilane, and its reaction is given below:
$nSi{{(C{{H}_{3}})}_{2}}C{{l}_{2}}+n{{H}_{2}}O\to {{[Si{{(C{{H}_{3}})}_{2}}O]}_{n}}+2nHCl$
Due to its electrical insulation property, it is used in a wide range of electrical appliances. It also has a very low toxicity level. It is also resistant to UV (ultra-violet) radiation, and ozone.
It is also used in the aerospace industry. It is an alternating dry cleaning solvent for the chlorine-containing perchloroethylene solvent.
Note:There are some silicone compounds like cyclic siloxanes which cause air and water pollution and sometimes cause negative health effects on the animals. Since it is used in medication, higher doses can cause serious health hazards.
Complete step-by-step answer:Polymer means there is a repeating unit called monomer which is repeated numerous times to form a macromolecule. Mostly the polymers are carbon-containing monomers, but there is another class of polymers called silicon polymer or polysiloxane. In these, the backbone chain is not formed by carbon atoms, it is made from silicon and oxygen atoms. Its monomer unit is called siloxane and it is represented as $(-{{R}_{2}}Si-O-Si{{R}_{2}}-)$, in which R is the alkyl group. The basic structure of silicon polymer is given below:

These polymers have many good and useful properties like they are water repellent, have heat stability, they have good resistance to chemical attack. These are used in many varieties like rubber-material, oils, grease, adhesives, medicines, cooking utensils, etc.
We can prepare a siloxane, polydimethylsiloxane by the hydrolysis of dimethyldichlorosilane, and its reaction is given below:
$nSi{{(C{{H}_{3}})}_{2}}C{{l}_{2}}+n{{H}_{2}}O\to {{[Si{{(C{{H}_{3}})}_{2}}O]}_{n}}+2nHCl$
Due to its electrical insulation property, it is used in a wide range of electrical appliances. It also has a very low toxicity level. It is also resistant to UV (ultra-violet) radiation, and ozone.
It is also used in the aerospace industry. It is an alternating dry cleaning solvent for the chlorine-containing perchloroethylene solvent.
Note:There are some silicone compounds like cyclic siloxanes which cause air and water pollution and sometimes cause negative health effects on the animals. Since it is used in medication, higher doses can cause serious health hazards.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




