
What do you mean by close packing?
Answer
539.7k+ views
Hint:The close packing structures are present in solids because the spaces between the solids are very less due to which the atoms are very close to each other. In close packing, there are many types like close packing in one-dimension, close packing in two-dimension, etc.
Complete step-by-step answer:Close packing is a type of arrangement of the atoms or molecules in the compound. This arrangement is present in solids, i.e., crystals. The close packing structures are present in solids because the spaces between the solids are very less due to which the atoms are very close to each other. To understand the close packing in the solids, the atoms are molecules that are considered spherical. The atoms and molecules are arranged in a cubical crystal in which they arrange themselves to occupy maximum space which makes the solids denser, stronger, and harder.
There are many types of close packing, like close-packing in one-dimension, close packing in two-dimension, etc.
The close packing in one-dimension is when the atoms or molecules are in a straight line, i.e., in a single row. This is shown below:
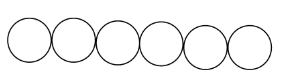
The close packing in two-dimension is when one layer of the molecules is over another layer. This is done in two ways when the molecule of one layer is exact over the other layer and it is called square close packing.

The other way is when the molecules of one layer are over the space between the molecules of the second layer and it is called hexagonal close packing. As shown below:
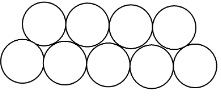
Note:As we can see from the diagrams that the space between the molecules in square close packing is more than the space between the hexagonal close packing.
Complete step-by-step answer:Close packing is a type of arrangement of the atoms or molecules in the compound. This arrangement is present in solids, i.e., crystals. The close packing structures are present in solids because the spaces between the solids are very less due to which the atoms are very close to each other. To understand the close packing in the solids, the atoms are molecules that are considered spherical. The atoms and molecules are arranged in a cubical crystal in which they arrange themselves to occupy maximum space which makes the solids denser, stronger, and harder.
There are many types of close packing, like close-packing in one-dimension, close packing in two-dimension, etc.
The close packing in one-dimension is when the atoms or molecules are in a straight line, i.e., in a single row. This is shown below:
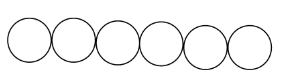
The close packing in two-dimension is when one layer of the molecules is over another layer. This is done in two ways when the molecule of one layer is exact over the other layer and it is called square close packing.

The other way is when the molecules of one layer are over the space between the molecules of the second layer and it is called hexagonal close packing. As shown below:

Note:As we can see from the diagrams that the space between the molecules in square close packing is more than the space between the hexagonal close packing.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




