
What are soaps and detergents?
Answer
588.9k+ views
Hint: Soap and detergent are derivatives of fatty acid chains in the form of chemical salt. The fatty acid chain belongs to the elements of the s-block group and the p-block group. Detergents are chemically synthesized products which are non-biodegradable.
Complete step by step answer:
Soaps: These are material used for the cleansing actions which are naturally obtained from fats and oils like from plants and animals. They act as mild surface-active agents.
Oils like vegetable oil, olive oil, coconut oil, etc are some from which oils are obtained.
Since they are made from a natural product, they are eco-friendly and biodegradable.
These are the slats of mainly sodium and potassium which belong to s-block elements.
The quality of the cleansing action of the soap is lesser than the detergents.
The reactivity of soap is less due to which they do not dissolve in hard water. Because hard water contains ions of calcium and magnesium. When the soap is dissolved in hard water it forms precipitate which is known as scum.
Some examples of soap are sodium stearate (${{C}_{18}}{{H}_{35}}Na{{O}_{2}}$), sodium palmitate (${{C}_{16}}{{H}_{31}}Na{{O}_{2}}$), etc.
Soap cannot be used in hard water because it forms scum due to the presence of bicarbonates, chlorides, and sulfates of calcium and magnesium in hard water.
Detergents: These are those materials that are used for the cleansing action that combines with oils, dirt, and impurities which make them more soluble in water.
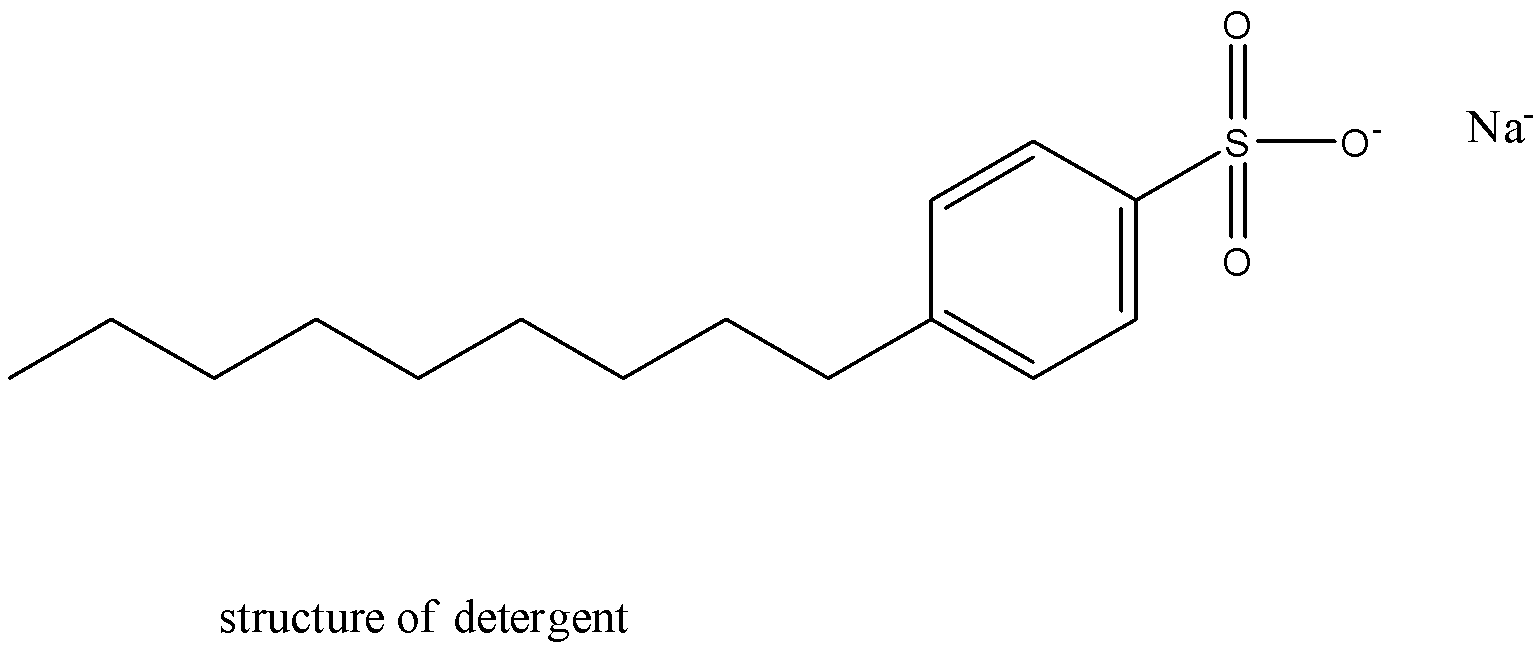
They are strong surface-active agents. They are alkylbenzene sulphonates.
They are not obtained from natural products instead they are produced chemically.
Since they are produced chemically they are less biodegradable because of the presence of the sulfonate group which does not get easily detached by the micro-organism in the sewage.
The quality of the cleansing action of detergent is higher than the soap.
The reactivity of detergent is then that's why they easily dissolve in soft as well as in hard water. Since they dissolve in hard water, there is no formation of scum.
These are ammonium or sulphonate salts with long-chain carboxylic acids.
Some examples of detergent are sodium lauryl sulfate ($Na{{C}_{12}}{{H}_{25}}S{{O}_{4}}$) and deoxycholic acid (${{C}_{24}}{{H}_{40}}{{O}_{4}}$), etc.
Note: Since both of them are used for cleaning, soaps can be used on our body because it is produced from natural products but detergent cannot be used on humans because they have high chemical content. Due to the high chemical content they can cause skin irritation, they can even be carcinogenic.
Complete step by step answer:
Soaps: These are material used for the cleansing actions which are naturally obtained from fats and oils like from plants and animals. They act as mild surface-active agents.
Oils like vegetable oil, olive oil, coconut oil, etc are some from which oils are obtained.
Since they are made from a natural product, they are eco-friendly and biodegradable.
These are the slats of mainly sodium and potassium which belong to s-block elements.
The quality of the cleansing action of the soap is lesser than the detergents.
The reactivity of soap is less due to which they do not dissolve in hard water. Because hard water contains ions of calcium and magnesium. When the soap is dissolved in hard water it forms precipitate which is known as scum.
Some examples of soap are sodium stearate (${{C}_{18}}{{H}_{35}}Na{{O}_{2}}$), sodium palmitate (${{C}_{16}}{{H}_{31}}Na{{O}_{2}}$), etc.
Soap cannot be used in hard water because it forms scum due to the presence of bicarbonates, chlorides, and sulfates of calcium and magnesium in hard water.
Detergents: These are those materials that are used for the cleansing action that combines with oils, dirt, and impurities which make them more soluble in water.
They are strong surface-active agents. They are alkylbenzene sulphonates.
They are not obtained from natural products instead they are produced chemically.
Since they are produced chemically they are less biodegradable because of the presence of the sulfonate group which does not get easily detached by the micro-organism in the sewage.
The quality of the cleansing action of detergent is higher than the soap.
The reactivity of detergent is then that's why they easily dissolve in soft as well as in hard water. Since they dissolve in hard water, there is no formation of scum.
These are ammonium or sulphonate salts with long-chain carboxylic acids.
Some examples of detergent are sodium lauryl sulfate ($Na{{C}_{12}}{{H}_{25}}S{{O}_{4}}$) and deoxycholic acid (${{C}_{24}}{{H}_{40}}{{O}_{4}}$), etc.

Note: Since both of them are used for cleaning, soaps can be used on our body because it is produced from natural products but detergent cannot be used on humans because they have high chemical content. Due to the high chemical content they can cause skin irritation, they can even be carcinogenic.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




