
What are diastereomers?
Answer
521.4k+ views
Hint: Stereoisomers are compounds with the same chemical formula and atom connectivity but a different three-dimensional shape or orientation. A stereocenter is a molecules atom (usually carbon) that is bonded to four separate atoms or groups of atoms.
Complete step by step answer:
Stereoisomers that are not mirror images of one another and cannot be superimposed over one another are called diastereomers. Diastereomers are stereoisomers that have two or more stereocenters. It can be difficult to tell whether two molecules are diastereomers or not.
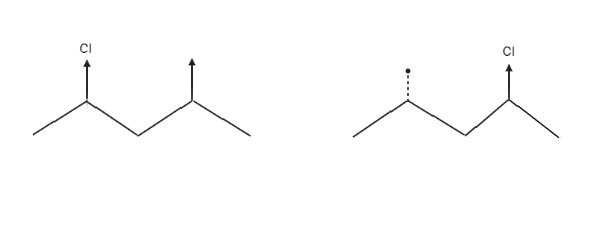
There are diastereomers, or molecules that are diametrically opposed. These molecules are not mirror images of one another and are non-superimposable, as seen below by flipping one of the molecules 180 degrees. The stereochemistry at the stereocenter attached to the methyl group is the same, whereas the stereochemistry at the other stereocenter attached to the chlorine is different.
Diastereomers often contain compounds with ring structures. Consider two six-membered ring compounds with two substituents, a chlorine atom, and an ethyl group in each. They are also not diastereomers because they are not mirror copies of each other. If a molecule has more than one chiral center, you run the risk of stereoisomers that do not match each other's pictures. Diastereomers are stereoisomers that are not mirror images of each other. When a molecule has two or more chiral centers, diastereomers can usually be observed.
Note:
Physical properties of diastereomers differ. Other than geometrical isomers, other diastereomers may or may not be optically active. Diastereomers have chemical properties that are similar but not identical. Techniques such as fractional crystallization, fractional distillation, and chromatography can be used to isolate diastereomers.
Complete step by step answer:
Stereoisomers that are not mirror images of one another and cannot be superimposed over one another are called diastereomers. Diastereomers are stereoisomers that have two or more stereocenters. It can be difficult to tell whether two molecules are diastereomers or not.

There are diastereomers, or molecules that are diametrically opposed. These molecules are not mirror images of one another and are non-superimposable, as seen below by flipping one of the molecules 180 degrees. The stereochemistry at the stereocenter attached to the methyl group is the same, whereas the stereochemistry at the other stereocenter attached to the chlorine is different.
Diastereomers often contain compounds with ring structures. Consider two six-membered ring compounds with two substituents, a chlorine atom, and an ethyl group in each. They are also not diastereomers because they are not mirror copies of each other. If a molecule has more than one chiral center, you run the risk of stereoisomers that do not match each other's pictures. Diastereomers are stereoisomers that are not mirror images of each other. When a molecule has two or more chiral centers, diastereomers can usually be observed.
Note:
Physical properties of diastereomers differ. Other than geometrical isomers, other diastereomers may or may not be optically active. Diastereomers have chemical properties that are similar but not identical. Techniques such as fractional crystallization, fractional distillation, and chromatography can be used to isolate diastereomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




