
Westron has the formula:-
(a)- $C{{F}_{2}}C{{l}_{2}}$
(b)- $CHC{{l}_{3}}$
(c)- ${{C}_{2}}{{H}_{2}}C{{l}_{4}}$
(d)- $CH{{F}_{3}}$
Answer
542.1k+ views
Hint: Westron is a chemical compound in which there is a total of 8 elements. There is an element in the Westron that belongs to group 17 and in period three of the periodic table that means the atomic number of the element is 17.
Complete step-by-step answer:Westron is an organic compound in which there is a total of 8 elements. There are three elements that in different ratios form the Westron compound. There are carbon atoms, hydrogen atoms, and chlorine atoms are present. There are 2 atoms of carbon element, 2 atoms of hydrogen element, and 4 atoms of chlorine element. So, the formula will be ${{C}_{2}}{{H}_{2}}C{{l}_{4}}$. Its IUPAC name is 1, 1, 2, 2-Tetrachloroethane. The structure of Westron is given below:
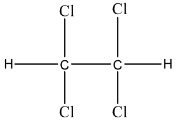
There are other names of Westron like TeCA, s-Tetrachloroethane, Acetylene tetrachloride, etc.
The molecular mass of Westron is 167.848 g/mol. The color of Westron lies between colorless to pale yellow and its physical state is the liquid state. Since this compound has chlorine atoms, therefore, its odor is pungent and chloroform-like.
The density of Westron is 1.59 $g/c{{m}^{3}}$.
The melting point of Westron is $-{{44}^{\circ }}C$ or 229 K and the boiling point is ${{146.5}^{\circ }}C$or 419.6 K.
The solubility of Westron in water is equal to 1g per 350mL.
In industries, the use of Westron is in the production of trichloroethylene, tetrachloroethylene, and 1, 2,-dichloroethylene.
Therefore, the correct answer is option (c)- ${{C}_{2}}{{H}_{2}}C{{l}_{4}}$.
Note: The Westron solvent is a very hazardous solvent that can cause many types of disease like Jaundice, enlarges the liver, headaches, tremors, dizziness, drowsiness, etc. This solvent is even banned in the United States.
Complete step-by-step answer:Westron is an organic compound in which there is a total of 8 elements. There are three elements that in different ratios form the Westron compound. There are carbon atoms, hydrogen atoms, and chlorine atoms are present. There are 2 atoms of carbon element, 2 atoms of hydrogen element, and 4 atoms of chlorine element. So, the formula will be ${{C}_{2}}{{H}_{2}}C{{l}_{4}}$. Its IUPAC name is 1, 1, 2, 2-Tetrachloroethane. The structure of Westron is given below:

There are other names of Westron like TeCA, s-Tetrachloroethane, Acetylene tetrachloride, etc.
The molecular mass of Westron is 167.848 g/mol. The color of Westron lies between colorless to pale yellow and its physical state is the liquid state. Since this compound has chlorine atoms, therefore, its odor is pungent and chloroform-like.
The density of Westron is 1.59 $g/c{{m}^{3}}$.
The melting point of Westron is $-{{44}^{\circ }}C$ or 229 K and the boiling point is ${{146.5}^{\circ }}C$or 419.6 K.
The solubility of Westron in water is equal to 1g per 350mL.
In industries, the use of Westron is in the production of trichloroethylene, tetrachloroethylene, and 1, 2,-dichloroethylene.
Therefore, the correct answer is option (c)- ${{C}_{2}}{{H}_{2}}C{{l}_{4}}$.
Note: The Westron solvent is a very hazardous solvent that can cause many types of disease like Jaundice, enlarges the liver, headaches, tremors, dizziness, drowsiness, etc. This solvent is even banned in the United States.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




