
Why is water not used as a barometric liquid. Give two reasons.
Answer
578.1k+ views
Hint: To answer the above question we first need to know what are the prerequisites for a substance that can be used as a barometric liquid. Further, we will verify whether water fulfills the required criteria. Once we know the above conditions we will accordingly verify as to why water cannot be used as the barometric liquid.
Complete step-by-step solution:
To begin with, let us understand what a barometer means. It is an instrument that we use to determine the atmospheric pressure.
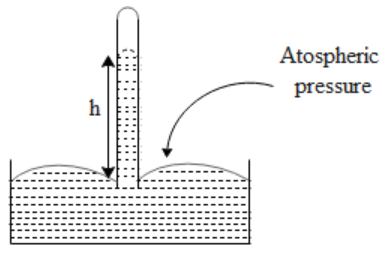
In the above diagram, we see a barometer, which consists of a tube and a container, which is filled with barometric liquid. Initially, we fill the tube till the brim, close it completely using the thumb, and then invert it. After inverting the liquid level comes down to some level. Let us now say the surface of the barometric liquid in the container is open to the atmosphere. If the density of the liquid is $\rho $and the liquid rises to a height “h” as shown in the figure, we can say that the atmospheric pressure(P) is equal to,
$P=\rho gh$ where g is the acceleration due to gravity.
The liquid is chosen such that it does not stick to the surface of the tube and expands uniformly with a change in temperature. But if we use water as a barometric liquid it will stick to the surface of the tube. Also, water does not show uniform expansion. Hence we can conclude that we cannot use water as a barometric liquid.
Note: Water has very strong adhesive forces than cohesive forces. Hence it usually sticks to the surface of any given material until and unless it's hydrophobic. In a barometer we use mercury as a barometric liquid; it fulfills all the criteria that are required by a substance to use it as the liquid in a barometer.
Complete step-by-step solution:
To begin with, let us understand what a barometer means. It is an instrument that we use to determine the atmospheric pressure.

In the above diagram, we see a barometer, which consists of a tube and a container, which is filled with barometric liquid. Initially, we fill the tube till the brim, close it completely using the thumb, and then invert it. After inverting the liquid level comes down to some level. Let us now say the surface of the barometric liquid in the container is open to the atmosphere. If the density of the liquid is $\rho $and the liquid rises to a height “h” as shown in the figure, we can say that the atmospheric pressure(P) is equal to,
$P=\rho gh$ where g is the acceleration due to gravity.
The liquid is chosen such that it does not stick to the surface of the tube and expands uniformly with a change in temperature. But if we use water as a barometric liquid it will stick to the surface of the tube. Also, water does not show uniform expansion. Hence we can conclude that we cannot use water as a barometric liquid.
Note: Water has very strong adhesive forces than cohesive forces. Hence it usually sticks to the surface of any given material until and unless it's hydrophobic. In a barometer we use mercury as a barometric liquid; it fulfills all the criteria that are required by a substance to use it as the liquid in a barometer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




