
Vinegar is a dilute aqueous solution of which acid.
Answer
507.6k+ views
Hint: Dilute acetic acid solution is called vinegar. The IUPAC name of acetic acid is ethanoic acid which contains carboxylic acid as a functional group. Acids are sour in taste and have a pungent smell.
Complete answer:
Vinegar is a dilute aqueous solution of acetic acid. As vinegar contains acid and thus it is sour in taste and has a pungent smell. Approximate 5 % of acetic acid is added into 95 % of water to make vinegar. The chemical formula of acetic acid is \[C{{H}_{3}}COOH\] and its structure is given as follows:
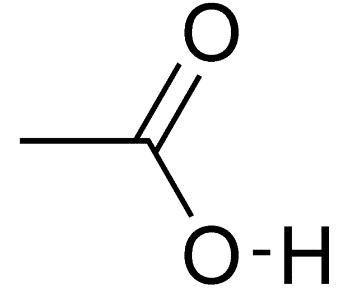
From the above structure, we conclude that carboxylic acid is present as a functional group in acetic acid. The IUPAC name of acetic acid is ethanoic acid.
Additional Information:
The word “Vinegar” is derived from latin in which “vin” refers to wine and “egar” refers to sour. Vinegar is commercially synthesized by the fermentation of sugar in the presence of yeast. Vinegar has antibacterial properties and that’s why it is used as a preservative in the kitchen in order to inhibit food spoilage. For instance, vinegar is used as a preservative in making pickles. Moreover, this antibacterial property of vinegar is also utilized in farms to inhibit bacterial and fungal growth.
Note:
It is important to note that vinegar is a dilute aqueous solution of acetic acid or ethanoic acid. Approximate 5 % of acetic acid is added into 95 % of water to make vinegar. Vinegar has a wide range of applications in everyday life especially in cooking, baking, in making pickles, in cleaning and so on.
Complete answer:
Vinegar is a dilute aqueous solution of acetic acid. As vinegar contains acid and thus it is sour in taste and has a pungent smell. Approximate 5 % of acetic acid is added into 95 % of water to make vinegar. The chemical formula of acetic acid is \[C{{H}_{3}}COOH\] and its structure is given as follows:

From the above structure, we conclude that carboxylic acid is present as a functional group in acetic acid. The IUPAC name of acetic acid is ethanoic acid.
Additional Information:
The word “Vinegar” is derived from latin in which “vin” refers to wine and “egar” refers to sour. Vinegar is commercially synthesized by the fermentation of sugar in the presence of yeast. Vinegar has antibacterial properties and that’s why it is used as a preservative in the kitchen in order to inhibit food spoilage. For instance, vinegar is used as a preservative in making pickles. Moreover, this antibacterial property of vinegar is also utilized in farms to inhibit bacterial and fungal growth.
Note:
It is important to note that vinegar is a dilute aqueous solution of acetic acid or ethanoic acid. Approximate 5 % of acetic acid is added into 95 % of water to make vinegar. Vinegar has a wide range of applications in everyday life especially in cooking, baking, in making pickles, in cleaning and so on.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




