
Using the Mulliken-barker test, how is the nitro group detected?
Answer
524.4k+ views
Hint : Mulliken barker test is used in the detecting nitro group in a compound. Nitro group is detected by the silver-mirror appearance that appears at the end of the reaction.
Complete Step By Step Answer:
In Mulliken barker test, the nitro group \[( - N{O_2})\] is reduced to hydroxylamine (usually known as \[HONO\] or \[ - NHOH\] group) in the presence of a neutral reducing agent. Hydroxylamine is further heated with Tollen’s reagent \[([Ag{(N{H_3})_2}]OH)\]. On heating, hydroxylamine gets oxidised to the respective nitroso compound and the Tollen’s reagent is reduced to metallic silver.
The following reaction will take place for Nitrobenzene in Mulliken-barker test:
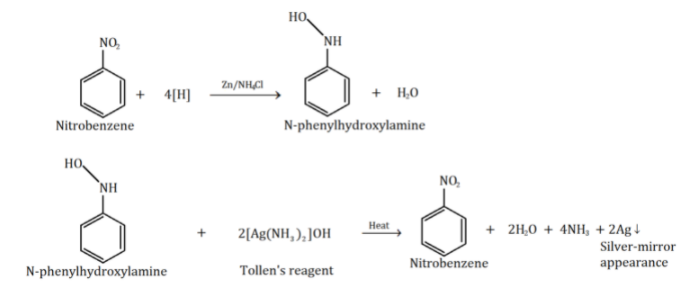
In the reaction above, nitrobenzene is first dissolved into dilute alcohol. Then, we add a solution of ammonium chloride along with zinc dust in the solution of dilute alcohol and nitrobenzene. This results in nitrobenzene turning to \[N - \] phenylhydroxylamine. This solution of \[N - \] phenylhydroxylamine is then filtered to another test tube where it is heated for \[2 - 3\] minutes. After that, a little amount of Tollen’s reagent is added into the solution. This gives a silver mirror appearance to the solution. This silver-mirror appearance is due to the precipitation of metallic silver.
Note :
This test can be used for all the compounds that contain nitro groups. The silver metal appearance obtained at the end of the reaction indicates the presence of nitro groups in the given compound. Tollen’s reagent is also used for testing the presence of aldehydes and ketones in a given solution. Tollen’s reagent oxidizes the given aldehyde into its corresponding carboxylic acid and gives a silver mirror appearance. While there is no reaction of Tollen’s reagent with ketones.
Complete Step By Step Answer:
In Mulliken barker test, the nitro group \[( - N{O_2})\] is reduced to hydroxylamine (usually known as \[HONO\] or \[ - NHOH\] group) in the presence of a neutral reducing agent. Hydroxylamine is further heated with Tollen’s reagent \[([Ag{(N{H_3})_2}]OH)\]. On heating, hydroxylamine gets oxidised to the respective nitroso compound and the Tollen’s reagent is reduced to metallic silver.
The following reaction will take place for Nitrobenzene in Mulliken-barker test:

In the reaction above, nitrobenzene is first dissolved into dilute alcohol. Then, we add a solution of ammonium chloride along with zinc dust in the solution of dilute alcohol and nitrobenzene. This results in nitrobenzene turning to \[N - \] phenylhydroxylamine. This solution of \[N - \] phenylhydroxylamine is then filtered to another test tube where it is heated for \[2 - 3\] minutes. After that, a little amount of Tollen’s reagent is added into the solution. This gives a silver mirror appearance to the solution. This silver-mirror appearance is due to the precipitation of metallic silver.
Note :
This test can be used for all the compounds that contain nitro groups. The silver metal appearance obtained at the end of the reaction indicates the presence of nitro groups in the given compound. Tollen’s reagent is also used for testing the presence of aldehydes and ketones in a given solution. Tollen’s reagent oxidizes the given aldehyde into its corresponding carboxylic acid and gives a silver mirror appearance. While there is no reaction of Tollen’s reagent with ketones.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




