
Use the bond energy data and calculate the enthalpy change for the above reaction. The bond energies of C-H and C-Cl are 413 and 328 kJ/ mol respectively.
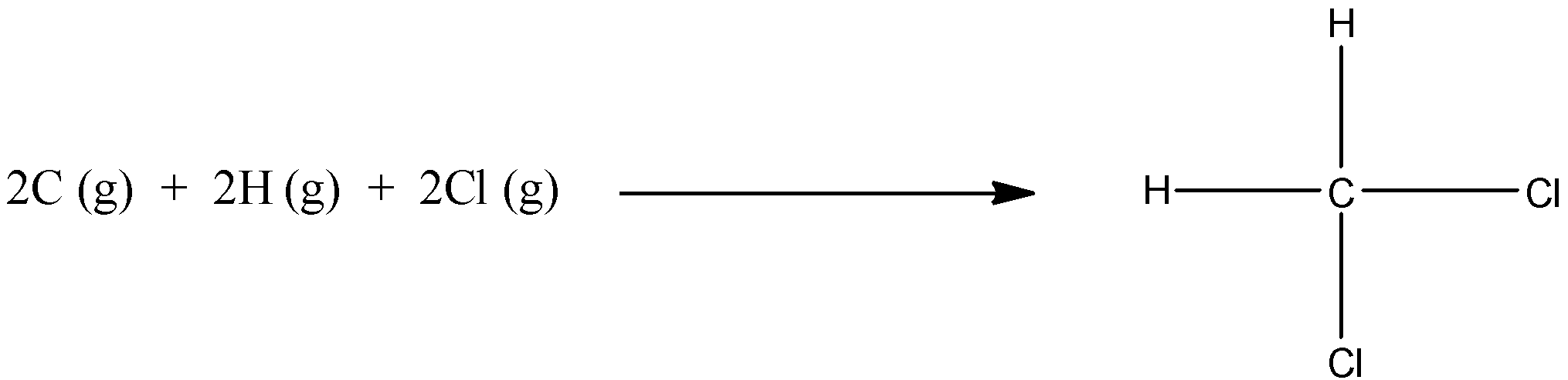
(a)- 1428 kJ/ mol
(b)- -1428 kJ/ mol
(c)- 2482 kJ/ mol
(d)- -2482 kJ/ mol
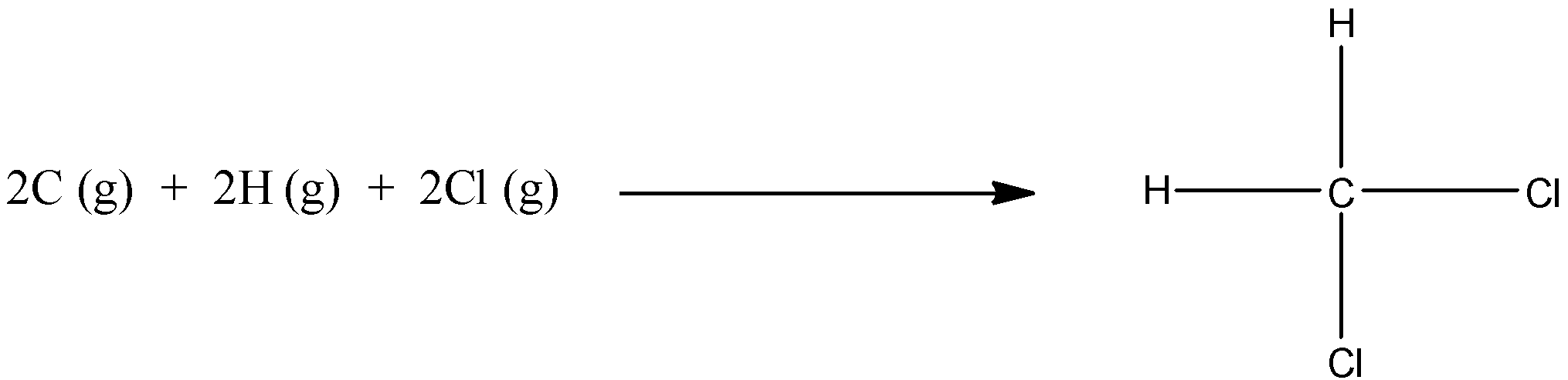
Answer
522.9k+ views
Hint: To calculate the change in enthalpy of the reaction, take the difference of the bond energies of the reactant and bond energies of the product. If there is no bond in reactant or product then, take the value as 0.
Complete answer:
We know that in the reaction, the reactant molecule combines with each other or there is a breaking of bond and the products are formed. New bonds are formed in products. Energy is either released or absorbed when the bond breaks or forms. With the help of bond energies, we can calculate the enthalpy of the reaction.
To calculate the change in enthalpy of the reaction, take the difference of the bond energies of the reactant and bond energies of the product. This can be written as:
$\Delta H=\sum{\text{Bond energy}{{\text{y}}_{R}}}-\sum{\text{Bond energy}{{\text{y}}_{P}}}$
The given reaction in the question is:
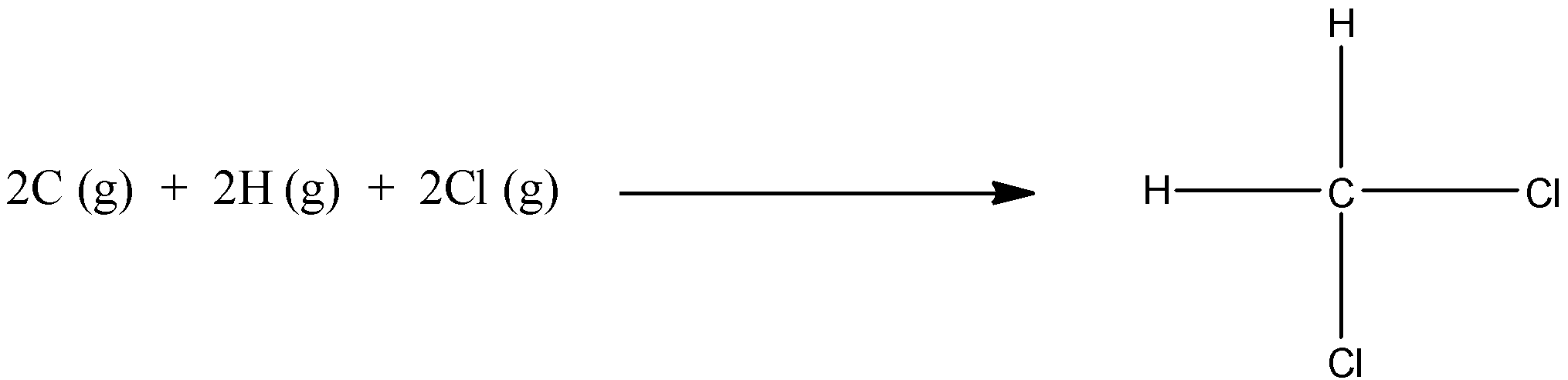
As we can see that there is no bond on the reactant side, so we have to take the value of bond energy as 0 for the reactant. On the product side, there are two C-H bonds and two C-Cl bonds, by adding their values, we get the bond energy of the product.
Now, putting all the values, we get:
$\Delta H=0-(413+413+328+328)=-1482$
So, the change in the enthalpy of the reaction is -1482 kJ/ mol.
Therefore, the correct answer is an option (b).
Note:
You have to add the bond energies of all the bonds present in the reaction. Sometimes, aromatic rings are given in the condensed form, so open all the structures which will help to calculate the correct bond energy.
Complete answer:
We know that in the reaction, the reactant molecule combines with each other or there is a breaking of bond and the products are formed. New bonds are formed in products. Energy is either released or absorbed when the bond breaks or forms. With the help of bond energies, we can calculate the enthalpy of the reaction.
To calculate the change in enthalpy of the reaction, take the difference of the bond energies of the reactant and bond energies of the product. This can be written as:
$\Delta H=\sum{\text{Bond energy}{{\text{y}}_{R}}}-\sum{\text{Bond energy}{{\text{y}}_{P}}}$
The given reaction in the question is:
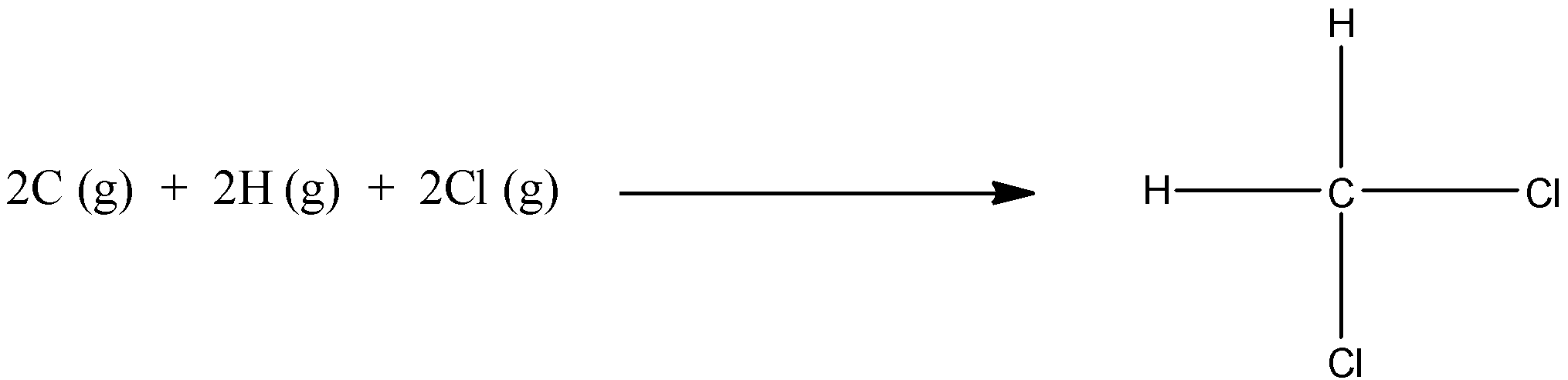
As we can see that there is no bond on the reactant side, so we have to take the value of bond energy as 0 for the reactant. On the product side, there are two C-H bonds and two C-Cl bonds, by adding their values, we get the bond energy of the product.
Now, putting all the values, we get:
$\Delta H=0-(413+413+328+328)=-1482$
So, the change in the enthalpy of the reaction is -1482 kJ/ mol.
Therefore, the correct answer is an option (b).
Note:
You have to add the bond energies of all the bonds present in the reaction. Sometimes, aromatic rings are given in the condensed form, so open all the structures which will help to calculate the correct bond energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




