
How many unpaired electrons are present in$N{i^{2 + }}$?
Answer
579.6k+ views
Hint: Nickel is a silvery metal which has a slight golden tinge. It is hard and ductile. It is corrosion resistant and heat resistant. It is a good conductor of electricity. It is also a ferromagnetic metal. It loses two electrons to form$N{i^{2 + }}$.
Complete step by step answer:
Nickel is a metal which has atomic number of nickel is $28$ and its electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2}$
This configuration is according to the decreasing order of energy of orbitals. We can represent the configuration of nickel as-
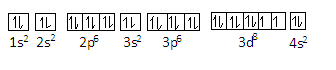
In this configuration nickel has $13$ paired electron pairs and two unpaired electrons (in the $3d$ orbital).
Nickel due to its heat resistance property is used to make alloys of great strength as well as heat, corrosion and oxidation resistant.
Now Nickel donates two electrons to form $N{i^{2 + }}$ ion. The reaction is given as-
$Ni \to N{i^{2 + }} + 2{e^ - }$
This means now the total number of electrons in the ion is $26$ as the element loses the two electrons of $4s$ orbital so the electronic configuration of $N{i^{2 + }}$ion is written as-
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}\]
We can represent the configuration of $N{i^{2 + }}$as-
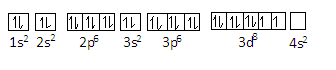
In this configuration, there are still $2$ unpaired electrons present in the $3d$ orbital.
The correct answer is $2$ unpaired electrons.
Note:
Nickel is used as-
-It is used in batteries
-It is used to plate other metals to protect them from corrosion.
-It is used to make alloys.
-It is used in toasters and electric ovens.
-It is used as a catalyst in many chemical reactions.
Complete step by step answer:
Nickel is a metal which has atomic number of nickel is $28$ and its electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2}$
This configuration is according to the decreasing order of energy of orbitals. We can represent the configuration of nickel as-

In this configuration nickel has $13$ paired electron pairs and two unpaired electrons (in the $3d$ orbital).
Nickel due to its heat resistance property is used to make alloys of great strength as well as heat, corrosion and oxidation resistant.
Now Nickel donates two electrons to form $N{i^{2 + }}$ ion. The reaction is given as-
$Ni \to N{i^{2 + }} + 2{e^ - }$
This means now the total number of electrons in the ion is $26$ as the element loses the two electrons of $4s$ orbital so the electronic configuration of $N{i^{2 + }}$ion is written as-
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}\]
We can represent the configuration of $N{i^{2 + }}$as-

In this configuration, there are still $2$ unpaired electrons present in the $3d$ orbital.
The correct answer is $2$ unpaired electrons.
Note:
Nickel is used as-
-It is used in batteries
-It is used to plate other metals to protect them from corrosion.
-It is used to make alloys.
-It is used in toasters and electric ovens.
-It is used as a catalyst in many chemical reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




