
How many unpaired electrons are in an atom of Co in its ground state?
A. $ 7 $
B. $ 1 $
C. $ 3 $
D. $ 2 $
Answer
492.9k+ views
Hint: In atomic or molecular orbitals, the distribution of electrons of an atom or molecule is the electron configuration. 27 is the atomic number of cobalt. Cobalt has the chemical symbol Co and belongs to the d-block of the periodic table. Cobalt in the periodic table is present in group 9 and period 4.
Complete Step By Step Answer:
Cobalt, a d-block element with symbol Co, has atomic number 27. The electronic configuration of cobalt is $ [Ar]3{d^7}4{s^2} $ . Electrons present in the outermost electronic configuration of an atom are the valence electrons.
9 is the valence electron in the cobalt atom. 7 electrons are present in the $ 3d $ orbital and 2 electrons in $ 4s $ orbital of the cobalt atom. The ground state molecular orbital structure of the cobalt atom will be:
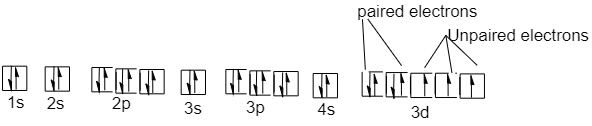
Electron filing takes place from lowest energy orbital to high energy orbital. In the d-orbital out of the 5 subshells, 2 subshells are totally occupied with two electrons of opposite spin, whereas the rest 3 subshells have only one electron each.
So cobalt has a total of 3 unpaired electrons present in its ground state.
Note:
Filling up of electrons in the orbitals of an atom is based on rules of the Aufbau principle, Pauli’s exclusion, and Hund’s rule. According to the Aufbau principle, the lower energy orbitals are filled first.
According to Pauli’s exclusion principle, only two electrons with opposite spins are allowed in an orbital, and Hund’s rule states that each orbital should be singly occupied in a sublevel before it is doubly occupied.
Complete Step By Step Answer:
Cobalt, a d-block element with symbol Co, has atomic number 27. The electronic configuration of cobalt is $ [Ar]3{d^7}4{s^2} $ . Electrons present in the outermost electronic configuration of an atom are the valence electrons.
9 is the valence electron in the cobalt atom. 7 electrons are present in the $ 3d $ orbital and 2 electrons in $ 4s $ orbital of the cobalt atom. The ground state molecular orbital structure of the cobalt atom will be:

Electron filing takes place from lowest energy orbital to high energy orbital. In the d-orbital out of the 5 subshells, 2 subshells are totally occupied with two electrons of opposite spin, whereas the rest 3 subshells have only one electron each.
So cobalt has a total of 3 unpaired electrons present in its ground state.
Note:
Filling up of electrons in the orbitals of an atom is based on rules of the Aufbau principle, Pauli’s exclusion, and Hund’s rule. According to the Aufbau principle, the lower energy orbitals are filled first.
According to Pauli’s exclusion principle, only two electrons with opposite spins are allowed in an orbital, and Hund’s rule states that each orbital should be singly occupied in a sublevel before it is doubly occupied.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




