
What do you understand by lone pair and shared pair?
Answer
503.7k+ views
Hint: Outer shell electrons which are associated with an atom and can participate in the formation of a chemical bond if the outer shell is not completely filled with electrons, then it is known as valence electron. In a single covalent bond, one valence electron is contributed by both atoms in the bonds to form a shared pair.
Complete answer:
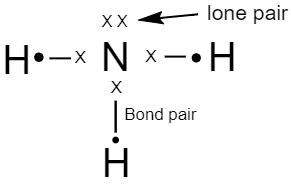
lone pair signifies a pair of valence electrons which are not shared with any of the other atoms in a covalent bond. The lone pair is also known as a non-bonded pair or asn unshared pair. Lone pairs help in explaining the shapes of molecules, the presence of lone pair decreases the bond angle between the bond pair of electrons because of their high electric charge that causes repulsion between the electrons. The lone pair also contributes to a molecule's dipole moment.
A covalent bond involves the sharing of electron pairs between atoms and these electron pairs are known as shared pairs or bonding pairs. In other words, the electron of a valence shell shared by two atoms to form a covalent bond is known as a shared pair of electrons.
In many molecules, the sharing of the electron allows each atom to attain the equivalent of a full valence shell, corresponding to stable electronic configuration.
Note:
The number of lone pair electrons + Number of bonding electrons = Total number of valence electrons around the atom.
Valence shell electron pair repulsion theory is used to predict the geometry of a molecule from the number of electron pairs surrounding the central atom. The valence electron pairs surrounding an atom tend to repel each other and then will adopt an arrangement which minimizes this repulsion.
Complete answer:
lone pair signifies a pair of valence electrons which are not shared with any of the other atoms in a covalent bond. The lone pair is also known as a non-bonded pair or asn unshared pair. Lone pairs help in explaining the shapes of molecules, the presence of lone pair decreases the bond angle between the bond pair of electrons because of their high electric charge that causes repulsion between the electrons. The lone pair also contributes to a molecule's dipole moment.
A covalent bond involves the sharing of electron pairs between atoms and these electron pairs are known as shared pairs or bonding pairs. In other words, the electron of a valence shell shared by two atoms to form a covalent bond is known as a shared pair of electrons.
In many molecules, the sharing of the electron allows each atom to attain the equivalent of a full valence shell, corresponding to stable electronic configuration.

Note:
The number of lone pair electrons + Number of bonding electrons = Total number of valence electrons around the atom.
Valence shell electron pair repulsion theory is used to predict the geometry of a molecule from the number of electron pairs surrounding the central atom. The valence electron pairs surrounding an atom tend to repel each other and then will adopt an arrangement which minimizes this repulsion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




