
How many uncharged resonance structures are there for azulene?
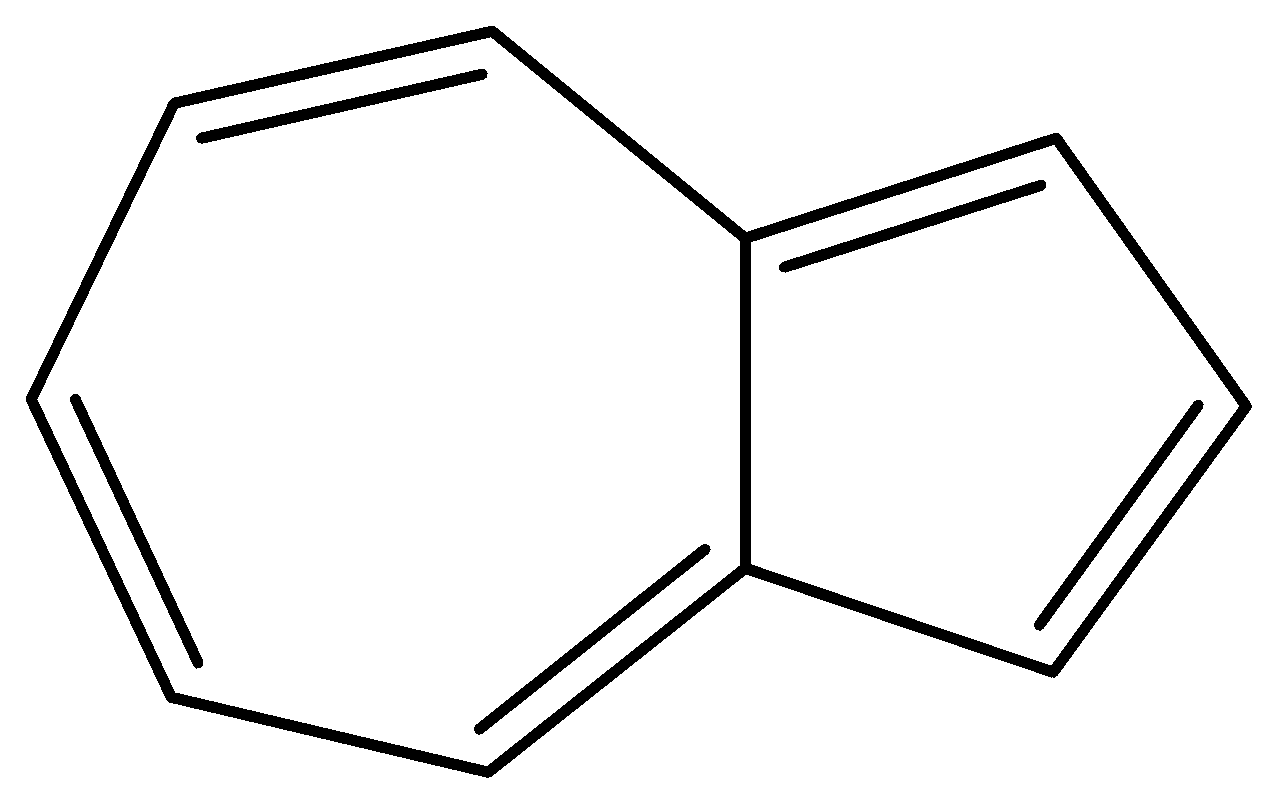
(A) 1
(B) 2
(C) 3
(D) 4

Answer
595.8k+ views
Hint: In chemistry, resonance is a way of describing bonding in certain organic molecules or ions by the combination of several contributing structures (or forms, also variously known as resonance structures or canonical structures) into a resonance hybrid (or hybrid structure) by shifting of carbon-carbon double bonds.
Complete step by step solution:
Azulene is a non-benzo combined ring system in which a seven membered ring of carbon atoms and a five membered ring of carbon atoms are fused together. The presence of $s{p^2}$hybridized carbon atoms facilitate the delocalization of electrons. This delocalisation of electrons then gives rise to various resonance structures of azulene.
There are a total five C=C bonds in an azulene molecule.
Let us now analyse the many resonance structures of Azulene before coming up with an answer.
In the various resonance structures for azulene, the most stable contributors put negative charge in the 5-membered ring and positive charge in the 7-membered ring.
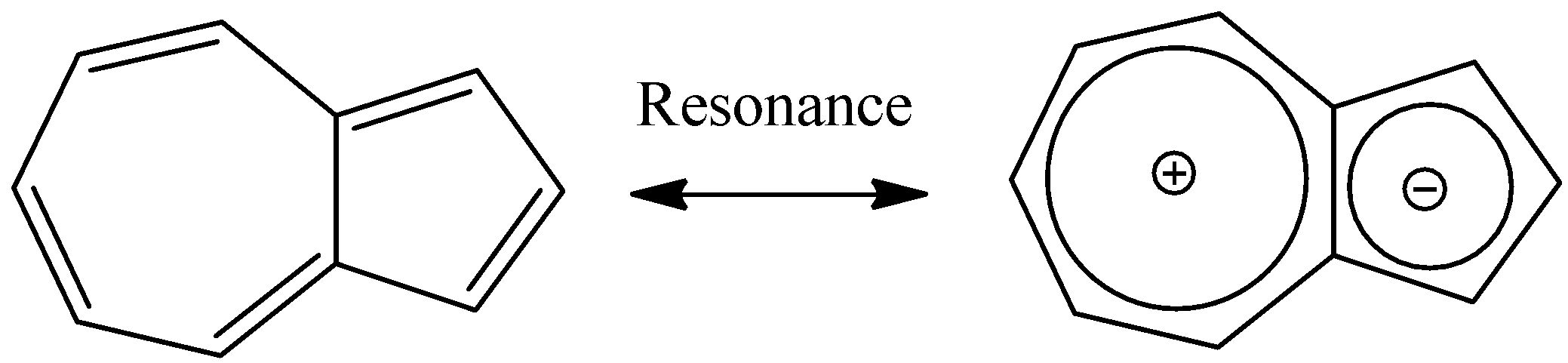
In effect, this gives an aromatic 6 π-electron five-membered ring fused to an aromatic 6 π-electron seven-membered ring, as we see in the above diagram.
However, this question concerns uncharged resonance structures, so let us now modify our approach so as to only be concerned with those structures of Azulene which are neutrally charged.
When trying to draw, uncharged resonance structures of the compound, we can only come up with the following:

So, only the way we can draw a resonance structure of azulene without charge is only this as shown above.
Therefore correct answer is (A)
Note: Make sure that Resonance structures should have the same number of electrons, do not add or subtract any electrons. Also ensure that each resonance structure follows the rules of writing Lewis Structures if any.
Complete step by step solution:
Azulene is a non-benzo combined ring system in which a seven membered ring of carbon atoms and a five membered ring of carbon atoms are fused together. The presence of $s{p^2}$hybridized carbon atoms facilitate the delocalization of electrons. This delocalisation of electrons then gives rise to various resonance structures of azulene.
There are a total five C=C bonds in an azulene molecule.
Let us now analyse the many resonance structures of Azulene before coming up with an answer.
In the various resonance structures for azulene, the most stable contributors put negative charge in the 5-membered ring and positive charge in the 7-membered ring.

In effect, this gives an aromatic 6 π-electron five-membered ring fused to an aromatic 6 π-electron seven-membered ring, as we see in the above diagram.
However, this question concerns uncharged resonance structures, so let us now modify our approach so as to only be concerned with those structures of Azulene which are neutrally charged.
When trying to draw, uncharged resonance structures of the compound, we can only come up with the following:

So, only the way we can draw a resonance structure of azulene without charge is only this as shown above.
Therefore correct answer is (A)
Note: Make sure that Resonance structures should have the same number of electrons, do not add or subtract any electrons. Also ensure that each resonance structure follows the rules of writing Lewis Structures if any.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




