
Ullmann reaction involves the use of the following reactants:
A) Iodobenzene and sodium
B) Benzene and copper
C) Iodobenzene and copper powder
D) Benzene diazonium chloride and Cu/HCl
Answer
587.7k+ views
Hint: Ullmann reaction, also known as Ullmann biaryl synthesis or Ullmann coupling, is an organic reaction which couples two molecules of aryl halide to produce a biaryl employing copper metal in the presence of thermal conditions.
Complete step by step answer:
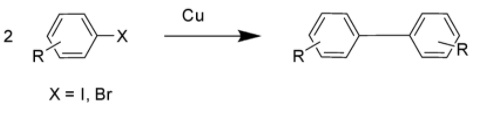
The general form of Ullmann reaction is depicted below:
Mechanism of Ullmann reaction: There are two well-known mechanisms for Ullmann reaction.
(i) The radical mechanism initiates with a single electron transfer from the copper metal to the alkyl halide forming an aryl radical. Then, two aryl radicals react to produce the biaryl product.
(ii) The second mechanism initiates with the oxidative addition of copper to the aryl halide which is then followed by a single electron transfer to produce an organocuprate reagent. Another oxidative addition occurs on an aryl halide by this organocuprate followed by reductive elimination, thus resulting in the biaryl product.
Step by step mechanism of Ullmann reaction:
1. Step 1:
When aryl halide is introduced to an excess of copper metal under thermal conditions (>200$^\circ$C), the Ullmann reaction leads to the production of an active copper(I) species.
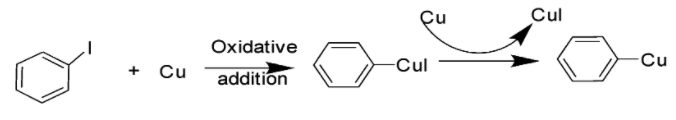
2. Step 2:
Now, the copper(I) species again undergoes oxidative addition with haloarene molecules thereby, linking the two molecules.
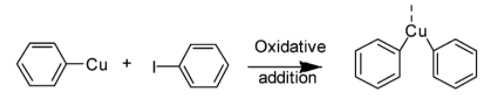
3. Step 3:
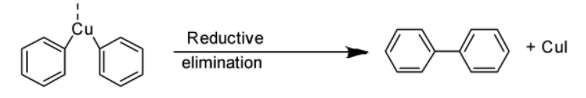
Finally, the copper compound produced by the two aryl halide molecules experiences reductive elimination, which results in the formation of a new carbon-carbon bond between the two aryl compounds (shown below).
Therefore, the answer to the above question is C.
Note: Ullmann coupling reaction is known to have relatively low yield that is why palladium-catalyzed coupling reactions (such as the Heck reaction) are preferred over this coupling reaction. Moreover, the reaction conditions for the Ullmann reaction are quite harsh.
Complete step by step answer:
The general form of Ullmann reaction is depicted below:

Mechanism of Ullmann reaction: There are two well-known mechanisms for Ullmann reaction.
(i) The radical mechanism initiates with a single electron transfer from the copper metal to the alkyl halide forming an aryl radical. Then, two aryl radicals react to produce the biaryl product.
(ii) The second mechanism initiates with the oxidative addition of copper to the aryl halide which is then followed by a single electron transfer to produce an organocuprate reagent. Another oxidative addition occurs on an aryl halide by this organocuprate followed by reductive elimination, thus resulting in the biaryl product.
Step by step mechanism of Ullmann reaction:
1. Step 1:
When aryl halide is introduced to an excess of copper metal under thermal conditions (>200$^\circ$C), the Ullmann reaction leads to the production of an active copper(I) species.

2. Step 2:
Now, the copper(I) species again undergoes oxidative addition with haloarene molecules thereby, linking the two molecules.

3. Step 3:
Finally, the copper compound produced by the two aryl halide molecules experiences reductive elimination, which results in the formation of a new carbon-carbon bond between the two aryl compounds (shown below).

Therefore, the answer to the above question is C.
Note: Ullmann coupling reaction is known to have relatively low yield that is why palladium-catalyzed coupling reactions (such as the Heck reaction) are preferred over this coupling reaction. Moreover, the reaction conditions for the Ullmann reaction are quite harsh.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




