
How many types of $ N - O $ bond lengths are present in:
$A)HN{O_3} \\
B)N{O_3}^ - $
Answer
515.7k+ views
Hint :we use the concept of resonance to explain the type of bonding present in $ HN{O_3} $ and $ N{O_3}^ - $ . In $ HN{O_3} $ the canonical forms show double-bond character in two bonds, causing them to be shorter than typical $ N - O $ bonds, that’s why the third $ N - O $ bond is elongated.
Complete Step By Step Answer:
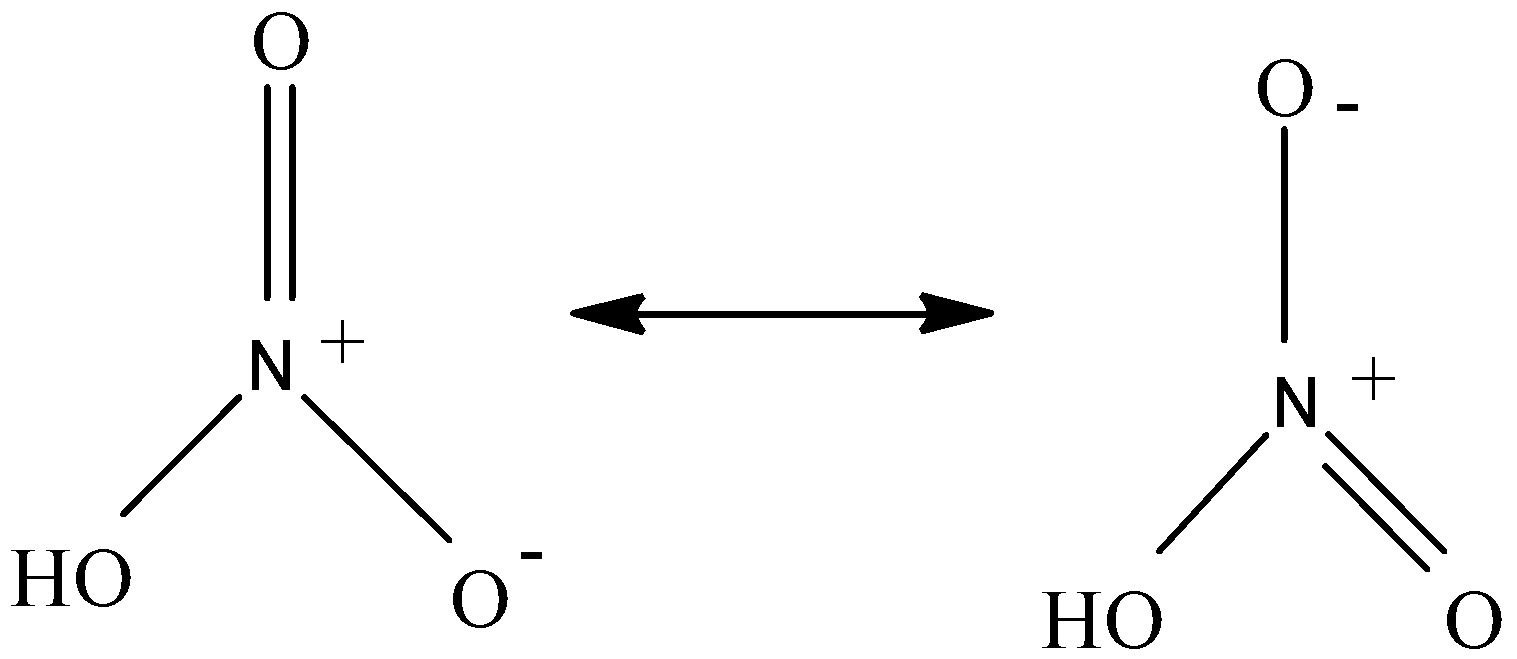
The lewis structure of nitric acid has a resonating structure. It has a $ \pi $ bond, covalent bond and a dative bond between $ N $ and $ O $ but no ionic bond is present.
The structure clearly shows that it has four single bonds and one $ \pi $ bond. Two of the $ N - O $ bonds are equivalent and relatively short, and the third $ N - O $ bond is elongated because the $ O $ atom is also attached to a proton.
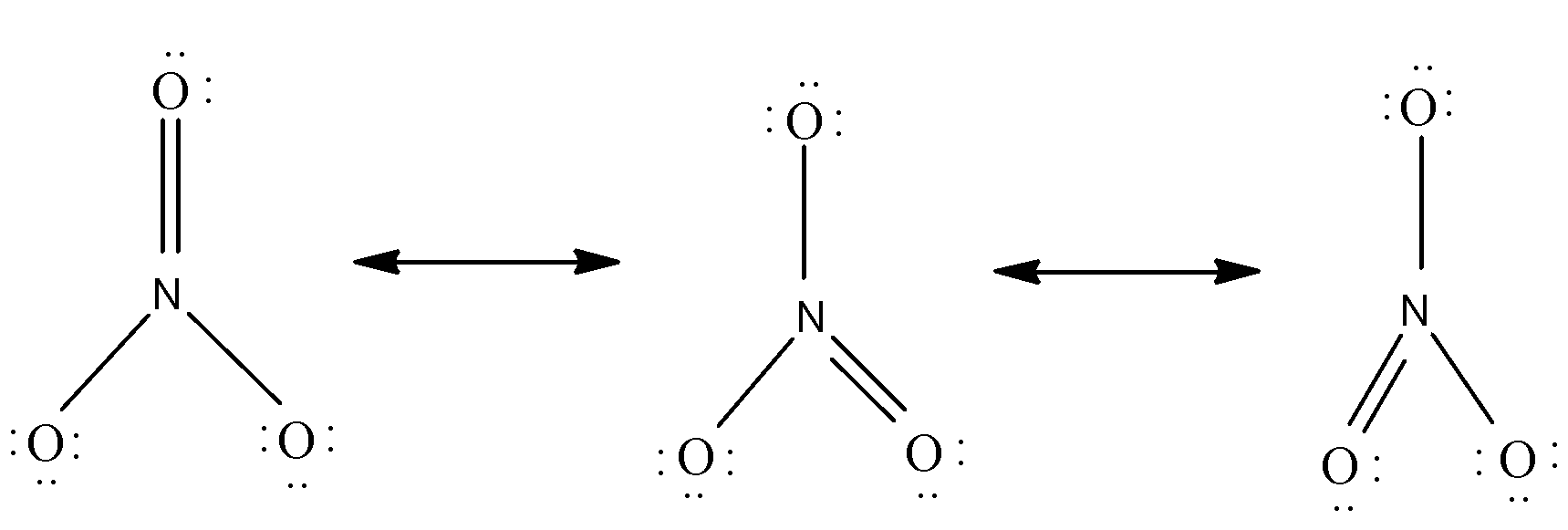
$ B) $ The nitrate ion, according to its Lewis diagram, has two types of nitrogen-oxygen bonds, one double bond and two single bonds, suggesting that one nitrogen-oxygen bond in the nitrate ion is shorter and stronger than each of the other two.
It consists of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. There are four $ \sigma $ and one $ \pi $ bond between $ N $ and $ O $ , so, it seems there are two covalent bonds and one double covalent bond.
Note :
Remember the nitrate ion carries a formal charge of $ - 1 $ . This charge results from a combination formal charge in which each of the three oxygens carries a $ - \dfrac{2}{3} $ charge, whereas the nitrogen carries a $ + 1 $ charge, all these adding up to formal charge of the polyatomic nitrate ion.
Complete Step By Step Answer:
The lewis structure of nitric acid has a resonating structure. It has a $ \pi $ bond, covalent bond and a dative bond between $ N $ and $ O $ but no ionic bond is present.
The structure clearly shows that it has four single bonds and one $ \pi $ bond. Two of the $ N - O $ bonds are equivalent and relatively short, and the third $ N - O $ bond is elongated because the $ O $ atom is also attached to a proton.

$ B) $ The nitrate ion, according to its Lewis diagram, has two types of nitrogen-oxygen bonds, one double bond and two single bonds, suggesting that one nitrogen-oxygen bond in the nitrate ion is shorter and stronger than each of the other two.
It consists of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. There are four $ \sigma $ and one $ \pi $ bond between $ N $ and $ O $ , so, it seems there are two covalent bonds and one double covalent bond.

Note :
Remember the nitrate ion carries a formal charge of $ - 1 $ . This charge results from a combination formal charge in which each of the three oxygens carries a $ - \dfrac{2}{3} $ charge, whereas the nitrogen carries a $ + 1 $ charge, all these adding up to formal charge of the polyatomic nitrate ion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




