
What type of isomers are \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\]?
A) Functional
B) Symmetrical
C) Configurational
D) Conformational
Answer
497.7k+ views
Hint: As we know that in organic chemistry isomers play a major role. In reaction time isomer plays an important role. Depending on the isomer of reactant only product will be obtained. In some cases equal amounts of two isomers will be obtained in the reaction. Acids and esters are called Tautomer. Alcohol and ethers are also called Tautomer. The tautomer isomers are commonly called functional isomers.
Complete answer:
The given molecular formula are \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\].
The molecular formula methyl ethyl ether is \[C{H_3}C{H_2}OC{H_3}\].
The molecular formula propanol is \[C{H_3}C{H_2}OC{H_3}\].
We can draw the structural formula methyl ethyl ether as,
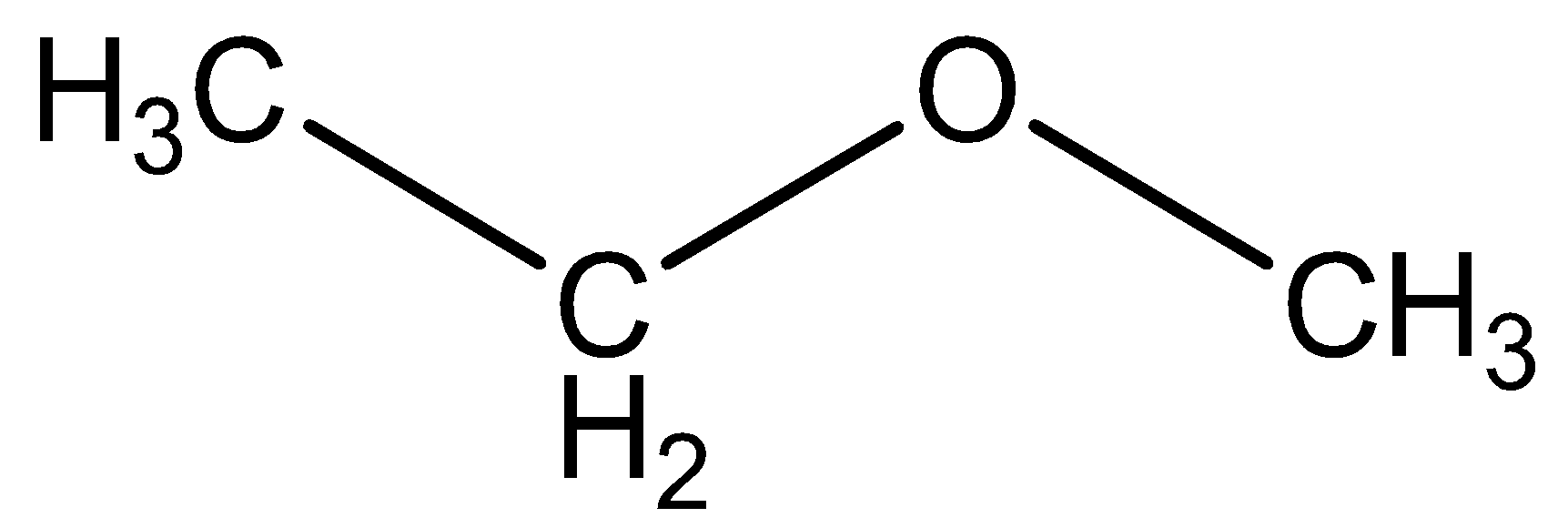
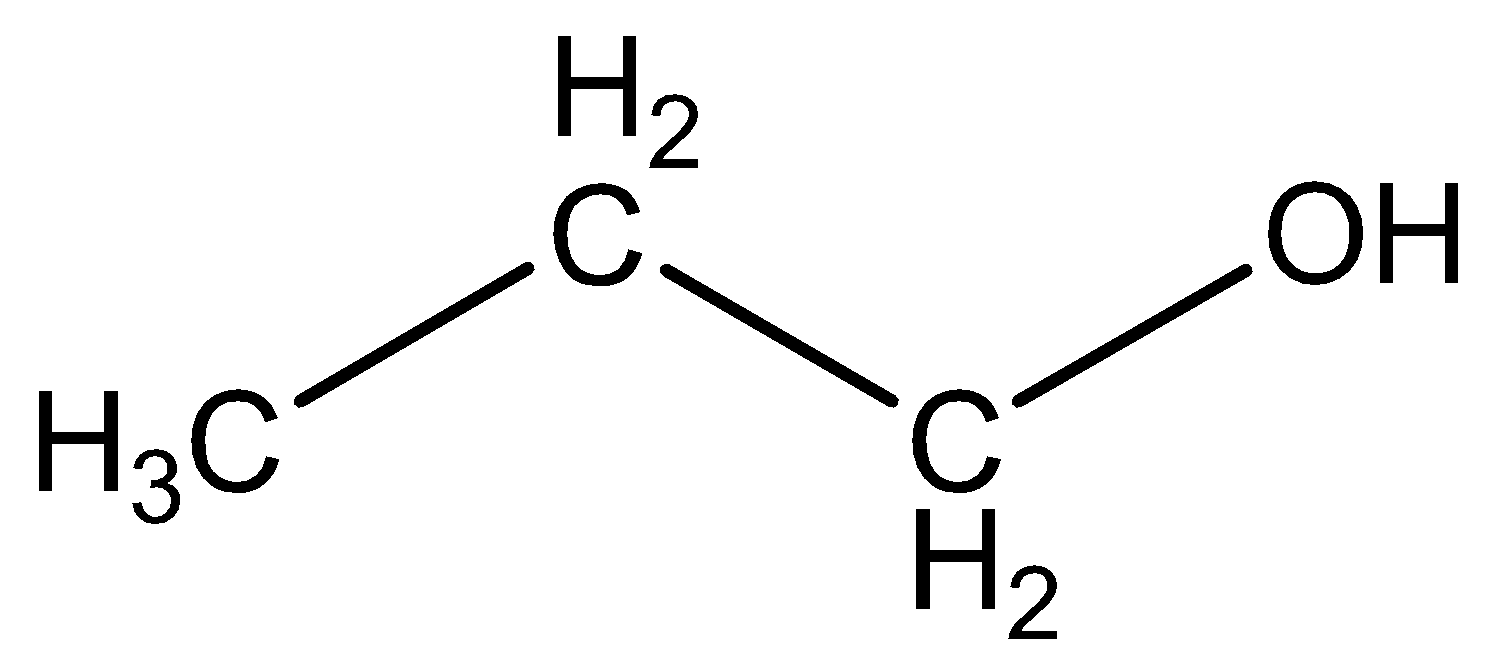
We can draw the structural formula propanol as,
The functional group in methyl ethyl ether \[C{H_3}C{H_2}OC{H_3}\] is ether.
The functional group in propanol \[C{H_3}C{H_2}OC{H_3}\] is alcohol.
The alcohol and ether are the functional isomers. So, \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\] are the functional isomers.
According to the above discussion, we conclude \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\] are the functional isomers.
Hence, option A is the correct answer.
Note:
We need to remember that the isomer of the organic molecule is divided by two types. There are constitutional isomers and stereoisomers. Further constitutional isomers are classified as chain isomer, position isomer, functional isomer, Metamers, Tautomer and ring chain isomer. The stereoisomers are classified as geometrical isomer and optical isomer. The isomer means the same molecular formula but different in structural or positional or functional or chain of the carbon atom in the organic molecule. Optical isomer should have a chiral carbon or asymmetric centre in the molecule. In organic chemistry isomers play a major role. In reaction time isomer plays an important role. Depending on the isomer of reactant only product will be obtained. In some cases equal amounts of two isomers will be obtained in the reaction.
Complete answer:
The given molecular formula are \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\].
The molecular formula methyl ethyl ether is \[C{H_3}C{H_2}OC{H_3}\].
The molecular formula propanol is \[C{H_3}C{H_2}OC{H_3}\].
We can draw the structural formula methyl ethyl ether as,

We can draw the structural formula propanol as,

The functional group in methyl ethyl ether \[C{H_3}C{H_2}OC{H_3}\] is ether.
The functional group in propanol \[C{H_3}C{H_2}OC{H_3}\] is alcohol.
The alcohol and ether are the functional isomers. So, \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\] are the functional isomers.
According to the above discussion, we conclude \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\] are the functional isomers.
Hence, option A is the correct answer.
Note:
We need to remember that the isomer of the organic molecule is divided by two types. There are constitutional isomers and stereoisomers. Further constitutional isomers are classified as chain isomer, position isomer, functional isomer, Metamers, Tautomer and ring chain isomer. The stereoisomers are classified as geometrical isomer and optical isomer. The isomer means the same molecular formula but different in structural or positional or functional or chain of the carbon atom in the organic molecule. Optical isomer should have a chiral carbon or asymmetric centre in the molecule. In organic chemistry isomers play a major role. In reaction time isomer plays an important role. Depending on the isomer of reactant only product will be obtained. In some cases equal amounts of two isomers will be obtained in the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




