
What type of intermolecular force of attraction is found in \[C{O_2}\] ?
Answer
534k+ views
Hint: Intermolecular forces refer to those forces that mediate interaction between the molecules and they include forces of attraction and repulsion which are supposed to act between the atoms or other neighbouring particles like atoms or ions. Different types of intermolecular forces include ionic bonds, Vander Waals dipole-dipole interaction, hydrogen bonding and Vander Waals dispersion forces.
Complete step by step answer:
Let us look at different intermolecular forces one by one and find out which kind of forces are present in \[C{O_2}\] .
The weakest kind of intermolecular forces are Vander Waals dispersion forces. As we already know that when atoms or molecules are polarizable to a certain degree, some interactions (Vander Waals dispersion forces) occur from them when they are brought together as electrons are pushed about. All atoms and molecules possess these Van der Waals forces, so these forces are also present in \[C{O_2}\] .
Dipole-dipole interactions are present between the polar molecules. Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. Its bond length is about$116.3pm$and has a linear structure. Its linear structure is supported by experimentally determined dipole moment of$0D$.
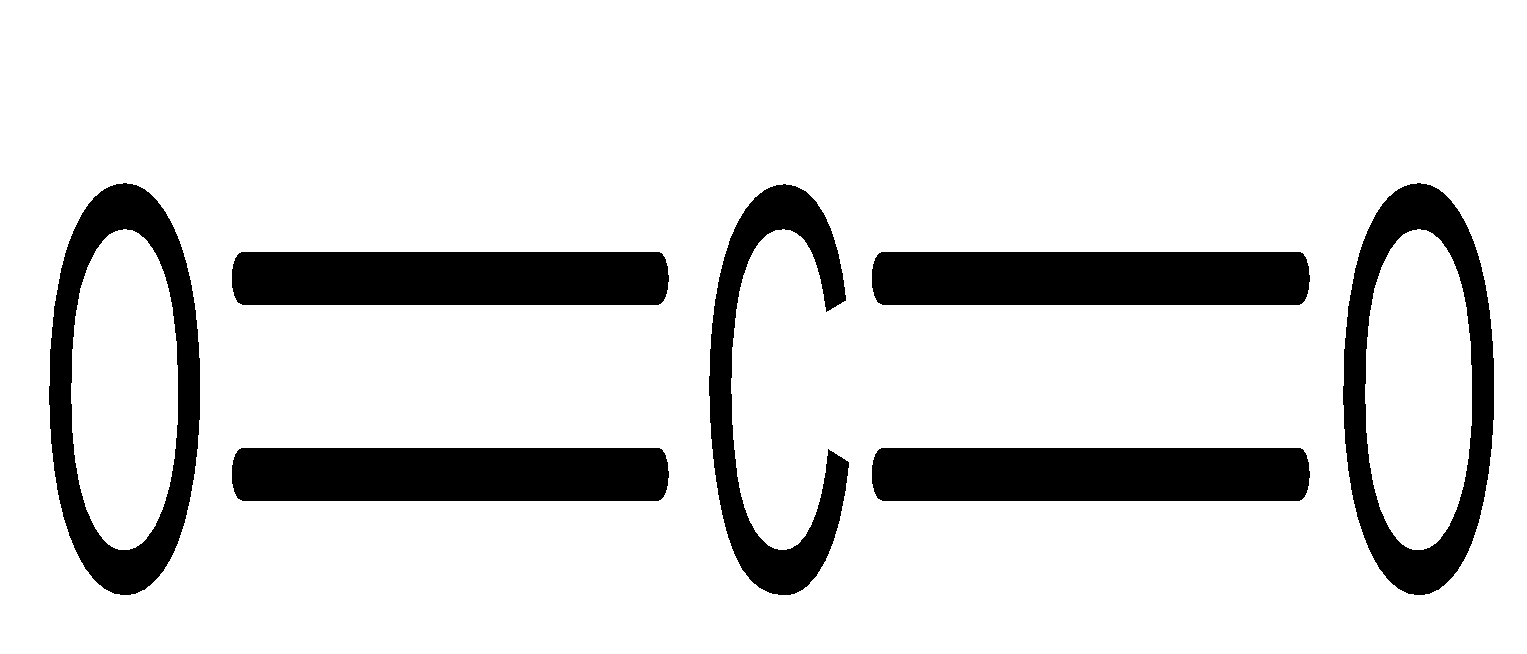
\[C{O_2}\] has two polar bonds (oxygen is more electronegative than carbon atom). But, dipoles in linear \[C{O_2}\] molecule cancel out each other, making \[C{O_2}\] non-polar. Thus, \[C{O_2}\] does not possess dipole-dipole interaction.
Hydrogen bonding occurs in those molecules where hydrogen atom is bonded to any of the three most electronegative atoms (which include \[N\] , \[O\] or \[F\] ) such that hydrogen atom possess partial positive charge, and also a lone pair of electron must be present on the electronegative atom. Since, \[C{O_2}\] does not contain any \[H\] atom, hydrogen bonding is not present in \[C{O_2}\] .
Hence, Vander Waals dispersion forces are found in \[C{O_2}\] .
Note: Vander Waals dispersion forces are stronger in molecules which are not compact, but possess long chains of elements. The reason is that it is more convenient and easier to displace the electrons as the forces of attraction between electrons and the protons in the nucleus are weaker.
Complete step by step answer:
Let us look at different intermolecular forces one by one and find out which kind of forces are present in \[C{O_2}\] .
The weakest kind of intermolecular forces are Vander Waals dispersion forces. As we already know that when atoms or molecules are polarizable to a certain degree, some interactions (Vander Waals dispersion forces) occur from them when they are brought together as electrons are pushed about. All atoms and molecules possess these Van der Waals forces, so these forces are also present in \[C{O_2}\] .
Dipole-dipole interactions are present between the polar molecules. Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. Its bond length is about$116.3pm$and has a linear structure. Its linear structure is supported by experimentally determined dipole moment of$0D$.
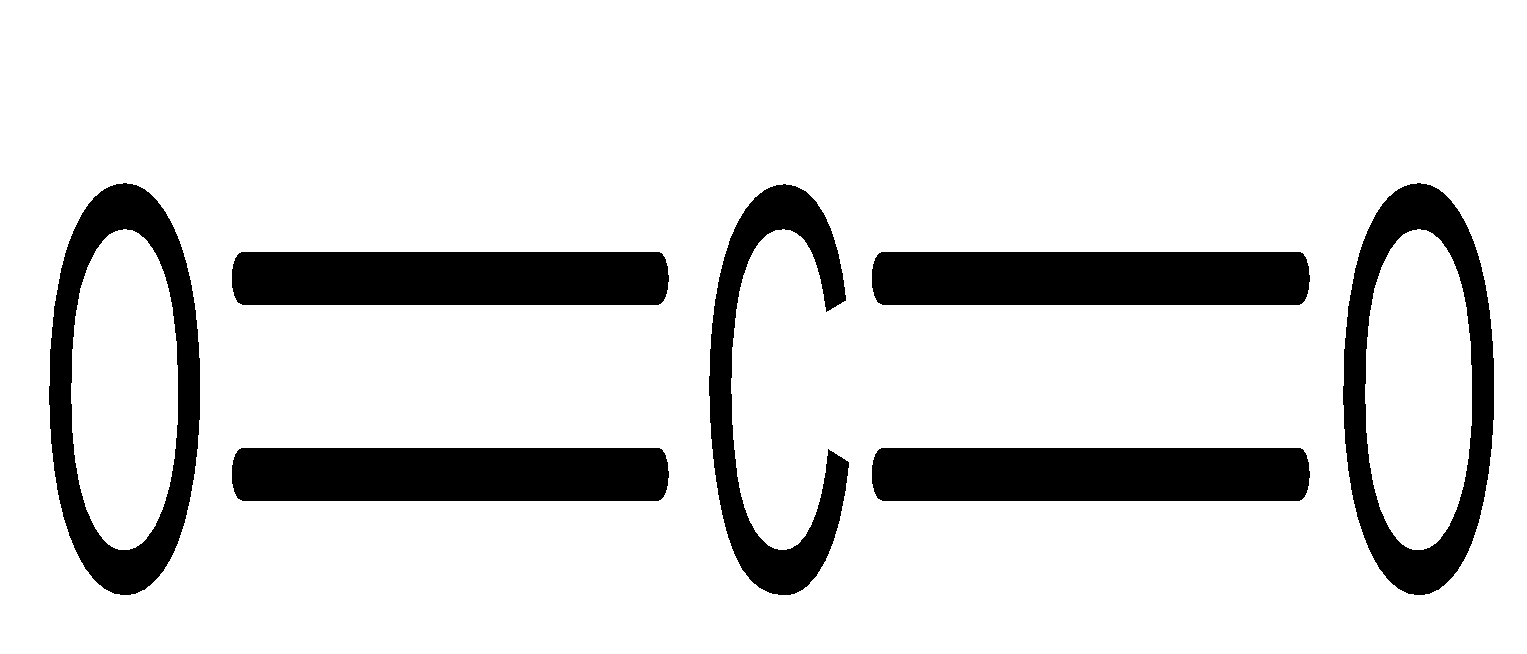
\[C{O_2}\] has two polar bonds (oxygen is more electronegative than carbon atom). But, dipoles in linear \[C{O_2}\] molecule cancel out each other, making \[C{O_2}\] non-polar. Thus, \[C{O_2}\] does not possess dipole-dipole interaction.
Hydrogen bonding occurs in those molecules where hydrogen atom is bonded to any of the three most electronegative atoms (which include \[N\] , \[O\] or \[F\] ) such that hydrogen atom possess partial positive charge, and also a lone pair of electron must be present on the electronegative atom. Since, \[C{O_2}\] does not contain any \[H\] atom, hydrogen bonding is not present in \[C{O_2}\] .
Hence, Vander Waals dispersion forces are found in \[C{O_2}\] .
Note: Vander Waals dispersion forces are stronger in molecules which are not compact, but possess long chains of elements. The reason is that it is more convenient and easier to displace the electrons as the forces of attraction between electrons and the protons in the nucleus are weaker.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




