
What is the type of hybridization in Ethane \[\left( {{C_2}{H_6}} \right)\]\[?\]
Answer
524.1k+ views
Hint: First we know hybridization is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theory. Then we know the types of hybridization. Then mention the type of hybridization in ethane with explanation.
Complete answer:
Consider the Lewis structure for Ethane \[\left( {{C_2}{H_6}} \right)\]
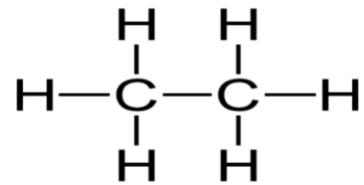
Here we can count \[4\] sigma bonds and \[0\] lone electron pairs (unshared electrons) around the central atom (carbon). Sigma bonds are overlaps of electron clouds between two atoms nuclei (a single bond). Pi bonds are bonds that do not get hybridized and are double and triple bonds. However, there is always 1 sigma bond in each double and triple bond.
The hybridization of sigma bonds and lone electron pairs goes as such:
1 cloud: \[sp\]
2 clouds: \[s{p^2}\]
3 clouds: \[s{p^3}\]
4 clouds: \[s{p^3}d\]
5 clouds (maximum): \[s{p^3}{d^2}\]
The four electron groups surrounding each carbon. It made four identical bonds in a perfect tetrahedral geometry, which means it needed four identical orbitals to make those bonds.
Each carbon has to hybridize one \[2s\] and three \[2p\] orbitals in order to generate four identical \[s{p^3}\]orbitals that are compatible in symmetry with hydrogen's \[1s\] orbitals.
Therefore, each \[C - H\] bond in \[\left( {{C_2}{H_6}} \right)\]is between an \[s{p^3}\] of carbon and a \[1s\] of hydrogen, i.e. an \[s{p^3}\]\[ - s\] connection, and each \[C - C\] bond is an \[s{p^3}\]-\[s{p^3}\] connection.
Hence, the type of hybridization of Ethane \[\left( {{C_2}{H_6}} \right)\]is \[s{p^3}\] hybridization.
Note:
Note that hybridization is also known as orbital hybridisation. Sigma bond is a chemical bond formed by the linear or co-axial overlapping of the atomic orbitals of two atoms. A pi bond is a type of covalent bond that exists between atoms where the electrons are on top and bottom of the axis connecting the nuclei of the joined atoms.
Complete answer:
Consider the Lewis structure for Ethane \[\left( {{C_2}{H_6}} \right)\]

Here we can count \[4\] sigma bonds and \[0\] lone electron pairs (unshared electrons) around the central atom (carbon). Sigma bonds are overlaps of electron clouds between two atoms nuclei (a single bond). Pi bonds are bonds that do not get hybridized and are double and triple bonds. However, there is always 1 sigma bond in each double and triple bond.
The hybridization of sigma bonds and lone electron pairs goes as such:
1 cloud: \[sp\]
2 clouds: \[s{p^2}\]
3 clouds: \[s{p^3}\]
4 clouds: \[s{p^3}d\]
5 clouds (maximum): \[s{p^3}{d^2}\]
The four electron groups surrounding each carbon. It made four identical bonds in a perfect tetrahedral geometry, which means it needed four identical orbitals to make those bonds.
Each carbon has to hybridize one \[2s\] and three \[2p\] orbitals in order to generate four identical \[s{p^3}\]orbitals that are compatible in symmetry with hydrogen's \[1s\] orbitals.
Therefore, each \[C - H\] bond in \[\left( {{C_2}{H_6}} \right)\]is between an \[s{p^3}\] of carbon and a \[1s\] of hydrogen, i.e. an \[s{p^3}\]\[ - s\] connection, and each \[C - C\] bond is an \[s{p^3}\]-\[s{p^3}\] connection.
Hence, the type of hybridization of Ethane \[\left( {{C_2}{H_6}} \right)\]is \[s{p^3}\] hybridization.
Note:
Note that hybridization is also known as orbital hybridisation. Sigma bond is a chemical bond formed by the linear or co-axial overlapping of the atomic orbitals of two atoms. A pi bond is a type of covalent bond that exists between atoms where the electrons are on top and bottom of the axis connecting the nuclei of the joined atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




