
What type of carbon-carbon bonds present in vanaspati ghee?
Answer
579k+ views
Hint: Vanaspati ghee is made from palm oil. It is prepared by hydrogenation of vegetable oils in the presence of nickel as a catalyst. Vanaspati ghee is a saturated hydrocarbon. The bonds present in saturated carbons are the type of bonds present in vanaspati ghee.
Complete step by step answer:
In India, vanaspati ghee is made from palm oil. Hydrogenation of vegetable oils in the presence of nickel which acts as a catalyst, at 473K temperature, and at low-medium pressure produces Vanaspati ghee. Hydrogenation is nothing but treating the compound with hydrogen.
The chemical formula of vanaspati is ${\left( {{C_{17}}{H_{33}}COO} \right)_3}{C_3}{H_5}$
$
Vegetable\, oil + {H_2}\xrightarrow{{Ni,473K}}Vanaspati \\
{\left( {{C_{17}}{H_{31}}COO} \right)_3}{C_3}{H_5} + 3{H_2}\xrightarrow{{Ni}}{\left( {{C_{17}}{H_{33}}COO} \right)_3}{C_3}{H_5} \\
$
Vanaspati ghee is a saturated hydrocarbon.
The bonds between carbon atoms are called covalent bonds. Saturated hydrocarbons only have carbon-carbon single bonds (C-C).
Unsaturated hydrocarbons are converted into saturated hydrocarbons by hydrogenation. For example, alkenes are converted into alkanes.
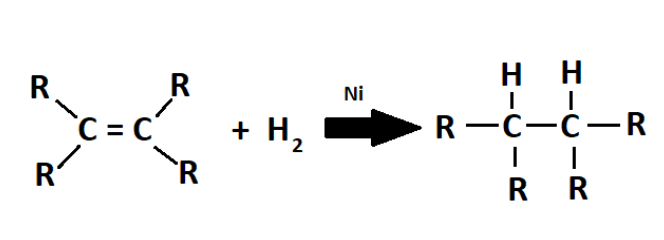
where R is any alkyl group
In the same way, vegetable oils which are unsaturated hydrocarbons are hydrogenated to form vanaspati ghee which is a saturated hydrocarbon.
Therefore, the type of carbon-carbon bonds present in Vanaspati ghee is a Single bond.
Additional Information: The catalyst used in the hydrogenation, nickel, does not react with the oil and hydrogen; it just increases the rate of the reaction.
Note:
In unsaturated carbons, carbon-carbon double bonds (C=C) and carbon-carbon triple bonds (C≡C) whereas saturated hydrocarbons carbon-carbon single bonds (C-C) are present. Do not confuse saturated hydrocarbons with unsaturated hydrocarbons. The IUPAC names of saturated compounds mostly end with “ane” and IUPAC names of unsaturated compounds end with “ene” and “yne”, for example, ethane is saturated and ethene, ethyne is unsaturated.
Complete step by step answer:
In India, vanaspati ghee is made from palm oil. Hydrogenation of vegetable oils in the presence of nickel which acts as a catalyst, at 473K temperature, and at low-medium pressure produces Vanaspati ghee. Hydrogenation is nothing but treating the compound with hydrogen.
The chemical formula of vanaspati is ${\left( {{C_{17}}{H_{33}}COO} \right)_3}{C_3}{H_5}$
$
Vegetable\, oil + {H_2}\xrightarrow{{Ni,473K}}Vanaspati \\
{\left( {{C_{17}}{H_{31}}COO} \right)_3}{C_3}{H_5} + 3{H_2}\xrightarrow{{Ni}}{\left( {{C_{17}}{H_{33}}COO} \right)_3}{C_3}{H_5} \\
$
Vanaspati ghee is a saturated hydrocarbon.
The bonds between carbon atoms are called covalent bonds. Saturated hydrocarbons only have carbon-carbon single bonds (C-C).
Unsaturated hydrocarbons are converted into saturated hydrocarbons by hydrogenation. For example, alkenes are converted into alkanes.

where R is any alkyl group
In the same way, vegetable oils which are unsaturated hydrocarbons are hydrogenated to form vanaspati ghee which is a saturated hydrocarbon.
Therefore, the type of carbon-carbon bonds present in Vanaspati ghee is a Single bond.
Additional Information: The catalyst used in the hydrogenation, nickel, does not react with the oil and hydrogen; it just increases the rate of the reaction.
Note:
In unsaturated carbons, carbon-carbon double bonds (C=C) and carbon-carbon triple bonds (C≡C) whereas saturated hydrocarbons carbon-carbon single bonds (C-C) are present. Do not confuse saturated hydrocarbons with unsaturated hydrocarbons. The IUPAC names of saturated compounds mostly end with “ane” and IUPAC names of unsaturated compounds end with “ene” and “yne”, for example, ethane is saturated and ethene, ethyne is unsaturated.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




