
Two oxides of Nitrogen, $NO$ and $N{{O}_{2}}$ react together at $253\;K$ and form a compound of Nitrogen $x$. $x$ reacts with water to yield another compound of Nitrogen $y$ . The shape of the anion of $y$ molecule is
a.Tetrahedral
b.Angular
c.Square planar
d.Pyramidal
Answer
527.4k+ views
Hint: The given two oxides for the given condition form a compound $x$ containing two nitrogen and three oxygen. This product reacts with water and forms acid of nitrogen $y$ . The anion of the acid will be an ion without the hydrogen ion. For the shape of the anion, the total number of bonds in the molecule will be giving a uniform shape.
Complete step by step answer:
Let us first construct the structural formula of the given compounds to understand their reaction easily.
The structural formula for $\;NO$ is as shown:
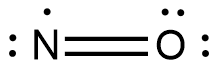
As the electron on nitrogen is unpaired, the compound is paramagnetic and the electron due to electronegativity does not remain localized and sometimes expressed as a dashed bond. The IUPAC name of this compound is Nitric Oxide.
Now, the structural formula for $N{{O}_{2}}$ is as shown:
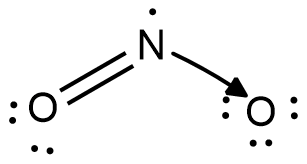
Here, the arrow from nitrogen towards oxygen signifies that the single nitrogen-oxygen bond is a coordinate covalent bond with both electrons of the bond belonging to nitrogen. The IUPAC name of this compound is Nitrogen Dioxide.
Because of the coordinate covalent bond, there is a partial positive charge on nitrogen and a partial negative charge on oxygen. Due to this, the double bond is a resonating bond and hence, in general structure both the nitrogen oxygen bonds are shown as a double bond with one thick line bond and one dashed line bond.
Now, the reaction of Nitric oxide and Nitrogen Dioxide at $253\;K$ will give product as shown
$NO+N{{O}_{2}}\to {{N}_{2}}{{O}_{3}}$
The IUPAC name of this product is Dinitrogen Trioxide. The structural formula for dinitrogen trioxide is as shown:
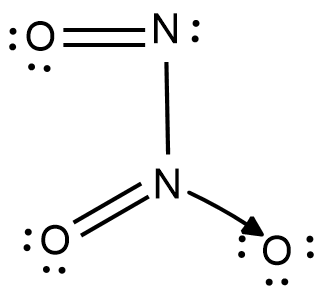
This is the compound $x$ as given in the data. This compound reacts with water, and the reaction can be expressed as
${{N}_{2}}{{O}_{3}}+{{H}_{2}}O\to 2HN{{O}_{2}}$
The IUPAC name of the product obtained is Nitrous acid. The structure of nitrous acid is as shown
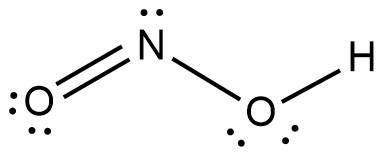
This is the compound $y$ as per the given data. The anion of this compound will be $N{{O}_{2}}^{-}$ and will be obtained by eliminating the hydrogen ion as shown
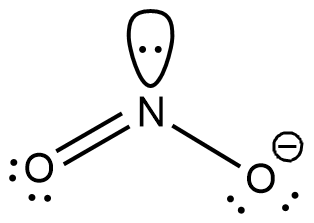
Now, for the shape of the molecule, we have to consider all the bonds as well as all the lone pairs on the parent atom.
From the above figure, we can say that there are a total of three bonds present. Hence, the shape of the molecule will be Triangular planar.
However, the X-rays cannot detect a lone pair of electrons. Hence, the X-ray image of the molecule will show the shape of the molecule as angular.
Hence, the correct answer is Option $(b)$
*The structural formulas shown above is to only show the bonding and the lone pair of electrons. The true bond lengths and bond angles might vary from the structure shown here.
Note:
When detecting the shape of a molecule, the total number of bonds in the molecule should include the number of lone pairs present on the parent atom. Thus, while considering the shape for a molecule, lone pairs are considered as bonds. For example, consider the molecule of water. The water molecule has two Oxygen-Hydrogen bonds as well as the parent atom oxygen has four extra electrons i.e. two pairs of lone pairs. Thus, it is considered to have four bonds and relatively its structure or shape is tetrahedral. But as the lone pairs are not seen in the X-ray image of the molecule, its shape is considered angular. Hence, the shape of the water molecule can be considered both angular as well as tetrahedral.
Complete step by step answer:
Let us first construct the structural formula of the given compounds to understand their reaction easily.
The structural formula for $\;NO$ is as shown:
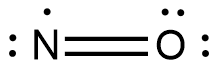
As the electron on nitrogen is unpaired, the compound is paramagnetic and the electron due to electronegativity does not remain localized and sometimes expressed as a dashed bond. The IUPAC name of this compound is Nitric Oxide.
Now, the structural formula for $N{{O}_{2}}$ is as shown:

Here, the arrow from nitrogen towards oxygen signifies that the single nitrogen-oxygen bond is a coordinate covalent bond with both electrons of the bond belonging to nitrogen. The IUPAC name of this compound is Nitrogen Dioxide.
Because of the coordinate covalent bond, there is a partial positive charge on nitrogen and a partial negative charge on oxygen. Due to this, the double bond is a resonating bond and hence, in general structure both the nitrogen oxygen bonds are shown as a double bond with one thick line bond and one dashed line bond.
Now, the reaction of Nitric oxide and Nitrogen Dioxide at $253\;K$ will give product as shown
$NO+N{{O}_{2}}\to {{N}_{2}}{{O}_{3}}$
The IUPAC name of this product is Dinitrogen Trioxide. The structural formula for dinitrogen trioxide is as shown:

This is the compound $x$ as given in the data. This compound reacts with water, and the reaction can be expressed as
${{N}_{2}}{{O}_{3}}+{{H}_{2}}O\to 2HN{{O}_{2}}$
The IUPAC name of the product obtained is Nitrous acid. The structure of nitrous acid is as shown

This is the compound $y$ as per the given data. The anion of this compound will be $N{{O}_{2}}^{-}$ and will be obtained by eliminating the hydrogen ion as shown

Now, for the shape of the molecule, we have to consider all the bonds as well as all the lone pairs on the parent atom.
From the above figure, we can say that there are a total of three bonds present. Hence, the shape of the molecule will be Triangular planar.
However, the X-rays cannot detect a lone pair of electrons. Hence, the X-ray image of the molecule will show the shape of the molecule as angular.
Hence, the correct answer is Option $(b)$
*The structural formulas shown above is to only show the bonding and the lone pair of electrons. The true bond lengths and bond angles might vary from the structure shown here.
Note:
When detecting the shape of a molecule, the total number of bonds in the molecule should include the number of lone pairs present on the parent atom. Thus, while considering the shape for a molecule, lone pairs are considered as bonds. For example, consider the molecule of water. The water molecule has two Oxygen-Hydrogen bonds as well as the parent atom oxygen has four extra electrons i.e. two pairs of lone pairs. Thus, it is considered to have four bonds and relatively its structure or shape is tetrahedral. But as the lone pairs are not seen in the X-ray image of the molecule, its shape is considered angular. Hence, the shape of the water molecule can be considered both angular as well as tetrahedral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




