
Why do two blocks of ice when put together and compressed become a single block of ice?
A. Ice blocks attract each other to form a bond
B. Melting point of ice increases when pressure is increased
C Melting point of ice decreases when pressure is increased
D. Latent heat of fusion of ice is high
Answer
478.5k+ views
Hint: We know that in normal conditions, melting point of ice is $0^\circ C$ . But when we apply pressure, the melting point changes and this results in the ice fusing. This is a complex mechanism, and will be discussed in greater depth.
Complete answer:
When excess amounts of pressure is applied on ice, it starts melting and then again refreezes when the pressure is removed.This phenomenon is known as the regelation of ice. The graph for melting point vs. pressure is given below.
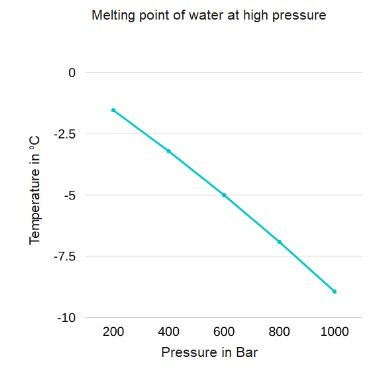
So, when you press two ice cubes together, due to excessive pressure, the ice at the ends melt and form a thin water layer between the contact surface of the two cubes.
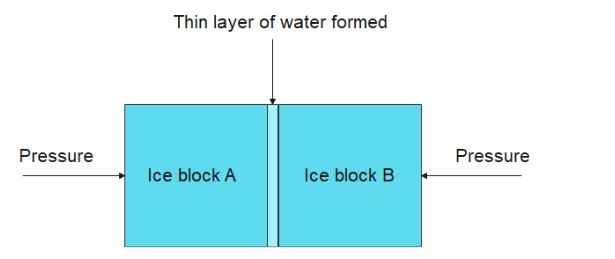
When this pressure is removed, the water layer freezes and causes the two blocks of ice to freeze.
Therefore, option C is the correct answer.
Note: Regelation of ice is a phenomenon studied under advanced physics. It involved advanced concepts such as surface melting, breaking and reformation of bonds and other complex mechanisms. In higher secondary level, a concise understanding of this concept is enough.
Complete answer:
When excess amounts of pressure is applied on ice, it starts melting and then again refreezes when the pressure is removed.This phenomenon is known as the regelation of ice. The graph for melting point vs. pressure is given below.

So, when you press two ice cubes together, due to excessive pressure, the ice at the ends melt and form a thin water layer between the contact surface of the two cubes.

When this pressure is removed, the water layer freezes and causes the two blocks of ice to freeze.
Therefore, option C is the correct answer.
Note: Regelation of ice is a phenomenon studied under advanced physics. It involved advanced concepts such as surface melting, breaking and reformation of bonds and other complex mechanisms. In higher secondary level, a concise understanding of this concept is enough.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




