
Truncated octahedral (cube-octahedra) is called ‘case’ or ‘sodalite cage’.
a.) True
b.) False
Answer
588.3k+ views
Hint: Truncated octahedral is structure formed from four and six tetrahedral structures. It is a ring structure composed of fusion of these four or six tetrahedral structures.
Complete step by step answer:
For this statement, first we will see what is a case or sodalite cage structure.
Sodalite is a cubic mineral. It forms a structure in which Na cations are in the interframework of aluminosilicate cage networks. The unit cell formed each consists of two cage structures.
The truncated octahedron is formed by fusion of rings of four and six tetrahedral. The linking in truncated octahedron form zeolite which has ‘case’ or ‘sodalite cage’ structure.
We can see that it looks somewhat like this.
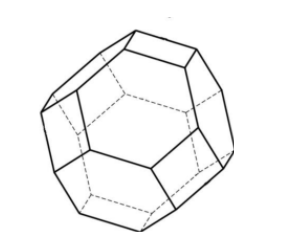
Thus, the statement given to us is true.
Additional Information:
This octahedron is an Archimedean solid. The structure is composed of 12 square faces, 8 regular hexagonal faces, 6 regular octagonal faces, 48 vertices and 72 edges.
This is even called a zonohedron because each face of the octahedron has point symmetry.
As it has foam-like tessellatory efficiency and permutohedral nature; thus, it can also be considered a three dimensional analogue of the hexagon.
We can even construct a truncated octahedron. It is done from a regular octahedron with side length 3a by the removal of six right square pyramids, each one from each point.
Note: Sodalite consists of chloride anions in the inter framework cages normally but in some structures, many other anions like hydroxide, sulphide etc. can also be seen. It is even known as an important container of many anions like iodide, iodate, perchlorate and permanganate etc.
Complete step by step answer:
For this statement, first we will see what is a case or sodalite cage structure.
Sodalite is a cubic mineral. It forms a structure in which Na cations are in the interframework of aluminosilicate cage networks. The unit cell formed each consists of two cage structures.
The truncated octahedron is formed by fusion of rings of four and six tetrahedral. The linking in truncated octahedron form zeolite which has ‘case’ or ‘sodalite cage’ structure.
We can see that it looks somewhat like this.

Thus, the statement given to us is true.
Additional Information:
This octahedron is an Archimedean solid. The structure is composed of 12 square faces, 8 regular hexagonal faces, 6 regular octagonal faces, 48 vertices and 72 edges.
This is even called a zonohedron because each face of the octahedron has point symmetry.
As it has foam-like tessellatory efficiency and permutohedral nature; thus, it can also be considered a three dimensional analogue of the hexagon.
We can even construct a truncated octahedron. It is done from a regular octahedron with side length 3a by the removal of six right square pyramids, each one from each point.
Note: Sodalite consists of chloride anions in the inter framework cages normally but in some structures, many other anions like hydroxide, sulphide etc. can also be seen. It is even known as an important container of many anions like iodide, iodate, perchlorate and permanganate etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




