
Trivial name of methanol is _____
A.Wood spirit
B.Methyl alcohol
C.Wood alcohol
D.All of the above
Answer
577.5k+ views
Hint: We have to know that trivial name means common name. We know that methanol is a primary alcohol that contains a methyl group linked to a hydroxyl group. We use methanol as a precursor for formaldehyde, methyl tert-butyl ether, acetic acid. We frequently abbreviate methanol as $MeOH$. The molar mass of methanol is \[32.04g/mol\].
Complete step by step answer:
We know that Methanol is an organic alcohol that has chemical formula $C{H_3}OH$. In methanol the methyl group is attached to a hydroxyl group. Methanol is a volatile, colourless, flammable liquid that has a smell similar to ethanol. Methanol is otherwise known as methyl alcohol.
Therefore, the option (A) is correct.
Methanol is produced chiefly by the destructive distillation of wood, and so it is also known as wood alcohol.
Therefore, the option (B) is also correct.
Methanol is also called as wood spirit, so option (C) is correct.
Since Options (A), (B), (C) are correct, all the above is the correct option.
Therefore, the option (D) is the final correct option.
We can draw the structure of methanol as,
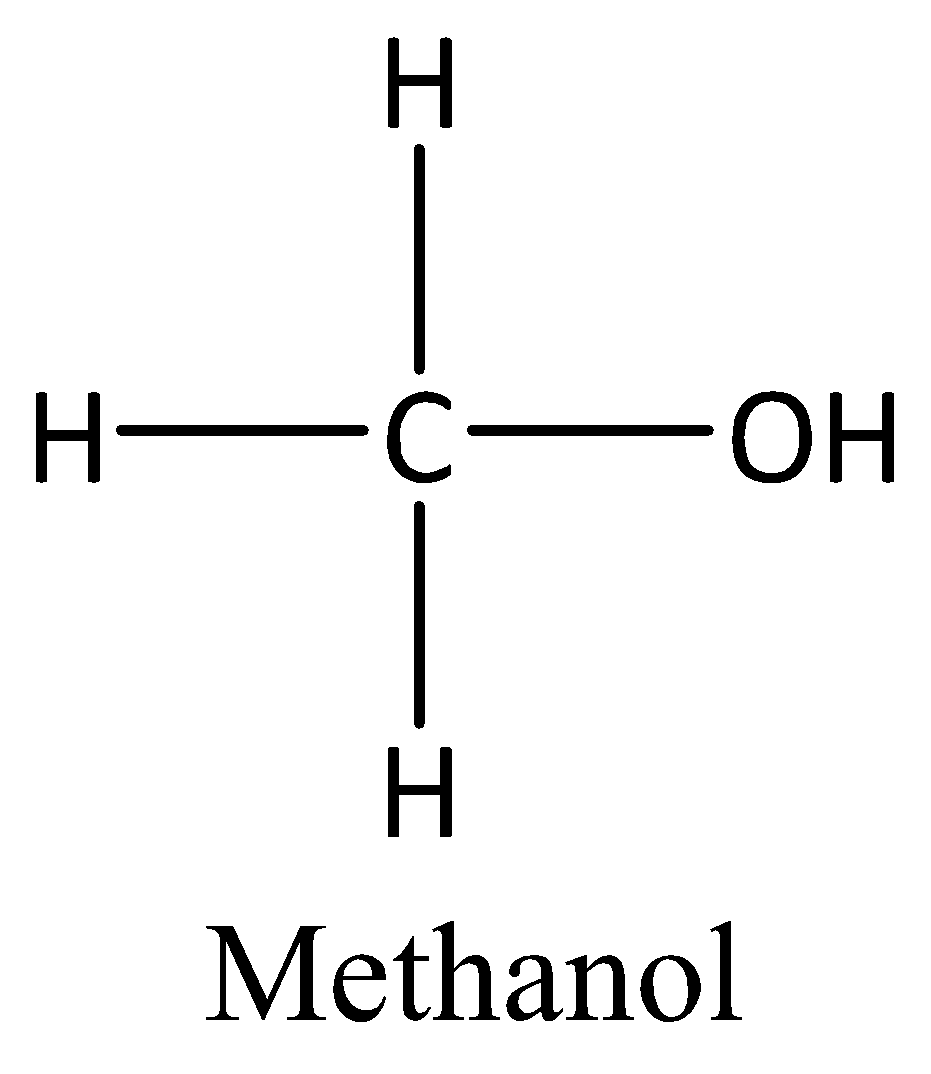
Industrially, we can prepare methanol by the hydrogenation of carbon monoxide. We can also produce methanol by anaerobic bacteria and phytoplankton.
So, the correct answer is Option D .
Note:
We can also write the chemical formula of methanol as $C{H_4}O$. We can obtain formaldehyde from methanol by oxidation reaction. We have to know that methanol undergoes a condensation reaction to form hydrocarbons. Methyl tert-butyl ether is obtained by the reaction of methanol with isobutene. We could also use methanol as fuel in internal combustion engines. Methanol forms water and carbon dioxide on combustion. Methanol could also be used as denaturant.
Complete step by step answer:
We know that Methanol is an organic alcohol that has chemical formula $C{H_3}OH$. In methanol the methyl group is attached to a hydroxyl group. Methanol is a volatile, colourless, flammable liquid that has a smell similar to ethanol. Methanol is otherwise known as methyl alcohol.
Therefore, the option (A) is correct.
Methanol is produced chiefly by the destructive distillation of wood, and so it is also known as wood alcohol.
Therefore, the option (B) is also correct.
Methanol is also called as wood spirit, so option (C) is correct.
Since Options (A), (B), (C) are correct, all the above is the correct option.
Therefore, the option (D) is the final correct option.
We can draw the structure of methanol as,

Industrially, we can prepare methanol by the hydrogenation of carbon monoxide. We can also produce methanol by anaerobic bacteria and phytoplankton.
So, the correct answer is Option D .
Note:
We can also write the chemical formula of methanol as $C{H_4}O$. We can obtain formaldehyde from methanol by oxidation reaction. We have to know that methanol undergoes a condensation reaction to form hydrocarbons. Methyl tert-butyl ether is obtained by the reaction of methanol with isobutene. We could also use methanol as fuel in internal combustion engines. Methanol forms water and carbon dioxide on combustion. Methanol could also be used as denaturant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




