
Trigonal bipyramidal geometry is shown by-
[A] $XeO{{F}_{2}}$
[B] $Xe{{O}_{3}}{{F}_{2}}$
[C] $FXeOS{{O}_{2}}F$
[D] ${{[Xe{{F}_{8}}]}^{2-}}$
Answer
597k+ views
Hint: To find the geometry of any complex, we need to determine the coordination number of the complex. If we know the coordination number, we can easily find out the hybridization and the geometry of the complex. The correct option is the anionic part of the complex.
Complete answer: Trigonal bipyramidal geometry is seen in complexes which have hybridization of$s{{p}^{3}}d$and their coordination number is 5.
Let us discuss each option to find out if they are in trigonal bipyramidal geometry or not-
In option [D], we have ${{[Xe{{F}_{8}}]}^{2-}}$
Number of valence electrons in Xe=8
Contribution of each fluorine atom is 1. Here we have 8 fluorine atoms therefore, for 8 fluorine atoms we will add 8.
We have an overall charge of -2, so we will add 2 to this.
The above mentioned addition will give us the total number of electrons. Dividing it by 2 will give us the coordination number-
$\therefore C.N=(8+8+2)\div 2$=9
Therefore, the coordination number of the complex is 9.
So, The hybridization=${{s}^{1}}{{p}^{3}}{{d}^{5}}$(1+2+5=9)
Shape of ${{[Xe{{F}_{8}}]}^{2-}}$ is square antiprism, which is difficult to draw on a 2d-plane.
In the next option, [C] we have, $FXeOS{{O}_{2}}F$
Number of valence electron on the central atom, Xe= 8
Contribution of each fluorine atom= 1
Contribution of Oxygen and sulphur is 0.
$\therefore C.N=(8+2)\div 2=5$
According to the calculation, the hybridization is $s{{p}^{3}}d$but this complex does not exist.
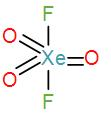
In option [B], we have $Xe{{O}_{3}}{{F}_{2}}$
Valence electrons in xenon=8
Contribution of each F atoms=2
$\therefore C.N=(8+2)\div 2=5$
Therefore, the geometry is trigonal bipyramidal. We can draw its structure as-
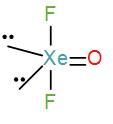
 Lastly, in option [A] we have, $XeO{{F}_{2}}$-
Lastly, in option [A] we have, $XeO{{F}_{2}}$-
Valence electrons in xenon=8
Contribution of each F atoms=2
$\therefore C.N=(8+2)\div 2=5$
Therefore, the geometry is trigonal bipyramidal. We can draw its structure as-
 Therefore, $Xe{{O}_{3}}{{F}_{2}}$and $XeO{{F}_{2}}$have trigonal bipyramidal geometry.
Therefore, $Xe{{O}_{3}}{{F}_{2}}$and $XeO{{F}_{2}}$have trigonal bipyramidal geometry.
Therefore, the correct options are-[A] $XeO{{F}_{2}}$and [B]$Xe{{O}_{3}}{{F}_{2}}$.
Note: Here, both $Xe{{O}_{3}}{{F}_{2}}$ and $XeO{{F}_{2}}$ have a trigonal bipyramidal geometry but they have dissimilar structures. $XeO{{F}_{2}}$ is T-shaped and it has 2 lone pairs but in $Xe{{O}_{3}}{{F}_{2}}$, all the electrons are occupied by oxygen and fluorine. Therefore, shape and geometry should be thought differently.
Complete answer: Trigonal bipyramidal geometry is seen in complexes which have hybridization of$s{{p}^{3}}d$and their coordination number is 5.
Let us discuss each option to find out if they are in trigonal bipyramidal geometry or not-
In option [D], we have ${{[Xe{{F}_{8}}]}^{2-}}$
Number of valence electrons in Xe=8
Contribution of each fluorine atom is 1. Here we have 8 fluorine atoms therefore, for 8 fluorine atoms we will add 8.
We have an overall charge of -2, so we will add 2 to this.
The above mentioned addition will give us the total number of electrons. Dividing it by 2 will give us the coordination number-
$\therefore C.N=(8+8+2)\div 2$=9
Therefore, the coordination number of the complex is 9.
So, The hybridization=${{s}^{1}}{{p}^{3}}{{d}^{5}}$(1+2+5=9)
Shape of ${{[Xe{{F}_{8}}]}^{2-}}$ is square antiprism, which is difficult to draw on a 2d-plane.
In the next option, [C] we have, $FXeOS{{O}_{2}}F$
Number of valence electron on the central atom, Xe= 8
Contribution of each fluorine atom= 1
Contribution of Oxygen and sulphur is 0.
$\therefore C.N=(8+2)\div 2=5$
According to the calculation, the hybridization is $s{{p}^{3}}d$but this complex does not exist.
In option [B], we have $Xe{{O}_{3}}{{F}_{2}}$
Valence electrons in xenon=8
Contribution of each F atoms=2
$\therefore C.N=(8+2)\div 2=5$
Therefore, the geometry is trigonal bipyramidal. We can draw its structure as-

Valence electrons in xenon=8
Contribution of each F atoms=2
$\therefore C.N=(8+2)\div 2=5$
Therefore, the geometry is trigonal bipyramidal. We can draw its structure as-

Therefore, the correct options are-[A] $XeO{{F}_{2}}$and [B]$Xe{{O}_{3}}{{F}_{2}}$.
Note: Here, both $Xe{{O}_{3}}{{F}_{2}}$ and $XeO{{F}_{2}}$ have a trigonal bipyramidal geometry but they have dissimilar structures. $XeO{{F}_{2}}$ is T-shaped and it has 2 lone pairs but in $Xe{{O}_{3}}{{F}_{2}}$, all the electrons are occupied by oxygen and fluorine. Therefore, shape and geometry should be thought differently.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




