
Transition metal compounds are usually colored. This is due to the electronic transition:
(a)- from p-orbital to s- orbital
(b)- from d- orbital to s- orbital
(c)- from p- orbital to p- orbital
(d)- within the d- orbital
Answer
590.1k+ views
Hint: The transition metal atoms belong to the d-block elements. The elements of d-block have incomplete d- orbitals due to which the compounds of transition metal are colored. The d- orbital splits into two sets.
Complete answer:
Most of the transition metal compounds(ionic as well as covalent) are colored both in the solid as well as in aqueous solution in contrast to the compounds of s- and p-block elements.
Color is due to the presence of incomplete d-subshell. Further, when the anion or the ligands approach the transition metal ions, their d-orbitals do not remain degenerated. They split into two sets, one consisting of lower energy orbitals (\[{{\text{t}}_{\text{2g}}}\text{which includes }{{\text{d}}_{\text{xy}}}\text{,}{{\text{d}}_{\text{yz}}}\text{,and }{{\text{d}}_{\text{zx}}}\]) and the other consisting of higher energy orbitals (\[{{e}_{\text{g}}}\text{which includes }{{\text{d}}_{{{\text{x}}^{2}}-{{y}^{2}}}}\text{,and }{{\text{d}}_{{{\text{z}}^{2}}}}\]). This is called crystal field splitting.
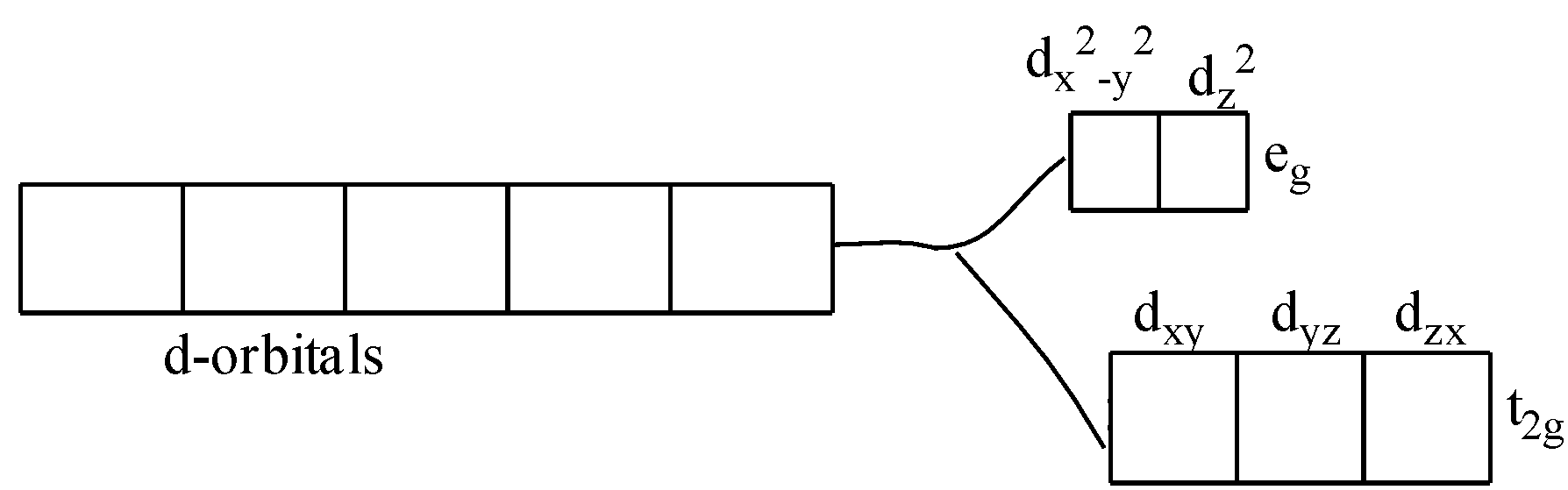
Thus, the electron can jump from lower energy d-orbital to higher energy d-orbital. The required amount of energy to do this is obtained by absorption of light of a particular wavelength in the region of visible light. One or more electrons are promoted from a lower level within the same d-orbital. The energy required to promote such an electron is very small. Radiation of light corresponding to such a small amount of energy is available within the visible region of light. The transition metal ion has the property to absorb such radiations from the visible light and appears colored due to the emission of the remainder as colored light.
Hence, the correct answer is an option (d)- within the d- orbital.
Note:
The ions and elements which have complete d-subshell or zero electrons in the d-subshell are colorless. This is because the electrons cannot jump from a lower level of d orbital to a higher level. Some examples of coloured compounds are: the compounds of copper are blue, the compounds of titanium are purple, etc.
Complete answer:
Most of the transition metal compounds(ionic as well as covalent) are colored both in the solid as well as in aqueous solution in contrast to the compounds of s- and p-block elements.
Color is due to the presence of incomplete d-subshell. Further, when the anion or the ligands approach the transition metal ions, their d-orbitals do not remain degenerated. They split into two sets, one consisting of lower energy orbitals (\[{{\text{t}}_{\text{2g}}}\text{which includes }{{\text{d}}_{\text{xy}}}\text{,}{{\text{d}}_{\text{yz}}}\text{,and }{{\text{d}}_{\text{zx}}}\]) and the other consisting of higher energy orbitals (\[{{e}_{\text{g}}}\text{which includes }{{\text{d}}_{{{\text{x}}^{2}}-{{y}^{2}}}}\text{,and }{{\text{d}}_{{{\text{z}}^{2}}}}\]). This is called crystal field splitting.

Thus, the electron can jump from lower energy d-orbital to higher energy d-orbital. The required amount of energy to do this is obtained by absorption of light of a particular wavelength in the region of visible light. One or more electrons are promoted from a lower level within the same d-orbital. The energy required to promote such an electron is very small. Radiation of light corresponding to such a small amount of energy is available within the visible region of light. The transition metal ion has the property to absorb such radiations from the visible light and appears colored due to the emission of the remainder as colored light.
Hence, the correct answer is an option (d)- within the d- orbital.
Note:
The ions and elements which have complete d-subshell or zero electrons in the d-subshell are colorless. This is because the electrons cannot jump from a lower level of d orbital to a higher level. Some examples of coloured compounds are: the compounds of copper are blue, the compounds of titanium are purple, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




