
Total number of lone pair of electrons in ${\text{XeO}}{{\text{F}}_{\text{4}}}$ is:
(A)- 0
(B)- 1
(C)- 2
(D)- 3
Answer
551.7k+ views
Hint: For calculating the number of lone pairs of electrons in any compound, first we have to know about the atomic number and electronic configuration of all the atoms present in that compound so that we will ensure the bonding between them.
Complete answer:
In the above given compound ${\text{XeO}}{{\text{F}}_{\text{4}}}$, xenon is the central atom as it is electropositive or less electronegative among oxygen and fluorine. For the calculation of lone pair of electron in ${\text{XeO}}{{\text{F}}_{\text{4}}}$ we will follow the following points:
-Atomic number of oxygen is 8 & its electronic configuration is written as ${\text{1}}{{\text{s}}^{\text{2}}}{\text{,2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{4}}}$ and from this it is clear that oxygen has total 6 valence electrons.
-Atomic number of fluorine is 9 & its electronic configuration is written as ${\text{1}}{{\text{s}}^{\text{2}}}{\text{,2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^5}$ and from this it is clear that oxygen has total 7 valence electrons.
-Atomic number of xenon is 54 & its electronic configuration is written as $\left[ {{\text{Kr}}} \right]{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{s}}^{\text{2}}}{\text{5}}{{\text{p}}^{\text{6}}}$ and from this it is clear that xenon has total 8 valence electrons. Among these 8 electrons 6 are used by the xenon for binding with fluorine and oxygen atoms in the following manner and remaining two electrons are present in the form of lone pair.
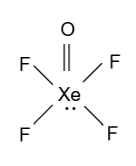
So in ${\text{XeO}}{{\text{F}}_{\text{4}}}$ one lone pair of electrons is present.
Hence, option (B) is correct.
Note:
Here some of you may do wrong if you count all the lone pairs of electrons of each atom present in the compound, because in this type of compound only lone pairs of central atoms are important, not of all atoms.
Complete answer:
In the above given compound ${\text{XeO}}{{\text{F}}_{\text{4}}}$, xenon is the central atom as it is electropositive or less electronegative among oxygen and fluorine. For the calculation of lone pair of electron in ${\text{XeO}}{{\text{F}}_{\text{4}}}$ we will follow the following points:
-Atomic number of oxygen is 8 & its electronic configuration is written as ${\text{1}}{{\text{s}}^{\text{2}}}{\text{,2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{4}}}$ and from this it is clear that oxygen has total 6 valence electrons.
-Atomic number of fluorine is 9 & its electronic configuration is written as ${\text{1}}{{\text{s}}^{\text{2}}}{\text{,2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^5}$ and from this it is clear that oxygen has total 7 valence electrons.
-Atomic number of xenon is 54 & its electronic configuration is written as $\left[ {{\text{Kr}}} \right]{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{s}}^{\text{2}}}{\text{5}}{{\text{p}}^{\text{6}}}$ and from this it is clear that xenon has total 8 valence electrons. Among these 8 electrons 6 are used by the xenon for binding with fluorine and oxygen atoms in the following manner and remaining two electrons are present in the form of lone pair.

So in ${\text{XeO}}{{\text{F}}_{\text{4}}}$ one lone pair of electrons is present.
Hence, option (B) is correct.
Note:
Here some of you may do wrong if you count all the lone pairs of electrons of each atom present in the compound, because in this type of compound only lone pairs of central atoms are important, not of all atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




