
To make the following reduction process spontaneous, temperature should be:
$ ZnO\, + \,C\,\xrightarrow{\Delta }\,Zn\, + \,CO $
(A) $ < 1000{\,^ \circ }\,C $
(B) $ > 1000{\,^ \circ }\,C $
(C) $ < 500{\,^ \circ }\,C $
(D) $ > 500{\,^ \circ }C\,but\, < \,{1000^ \circ }C $

Answer
539.4k+ views
Hint :This is Ellingham diagram in which spontaneity of any reaction is predicted using graph lines and temperature conditions. So, here see which graph lines are above and then place perpendicular lines along x and y axis by which we get to know the temperature conditions. As it was asked for a zinc reaction with oxygen see its graph line.
Complete Step By Step Answer:
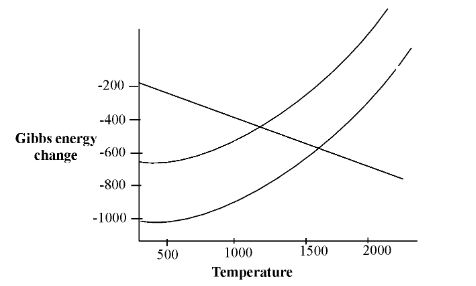
Here, as you see that the graph lines are given for a particular reaction. There are three types of reactions in the graph, first is the reaction of carbon with oxygen which will form the carbon monoxide having negative slope. In the second reaction, zinc is reacting with oxygen to give zinc oxide and the third one is the reaction of magnesium with oxygen giving magnesium oxide.
In Ellingham diagram questions you have to see the positive slope of the zinc reaction and thus as it is cutting carbon monoxide graph lines thus we should be able to make a feasible process above $ > 1000{\,^ \circ }\,C $ .
For spontaneous reactions which are feasible must have their Gibbs energy sign as negative, so for the conversion of zinc into zinc oxide is feasible spontaneous process when the reaction gets temperature above $ > 1000{\,^ \circ }\,C $ this kind of high temperature is provided by blast furnace.
Note :
We are seeing conversion of magnesium into magnesium oxide line also have positive graph slope hence if we try to find its temperature by where it becomes a spontaneous process, the temperature should be greater than $ 1500{\,^ \circ }\,C $ according to the Ellingham diagram, you will find it when you see that it is intercepting by line of carbon monoxide.
Complete Step By Step Answer:
Here, as you see that the graph lines are given for a particular reaction. There are three types of reactions in the graph, first is the reaction of carbon with oxygen which will form the carbon monoxide having negative slope. In the second reaction, zinc is reacting with oxygen to give zinc oxide and the third one is the reaction of magnesium with oxygen giving magnesium oxide.
In Ellingham diagram questions you have to see the positive slope of the zinc reaction and thus as it is cutting carbon monoxide graph lines thus we should be able to make a feasible process above $ > 1000{\,^ \circ }\,C $ .
For spontaneous reactions which are feasible must have their Gibbs energy sign as negative, so for the conversion of zinc into zinc oxide is feasible spontaneous process when the reaction gets temperature above $ > 1000{\,^ \circ }\,C $ this kind of high temperature is provided by blast furnace.
Note :
We are seeing conversion of magnesium into magnesium oxide line also have positive graph slope hence if we try to find its temperature by where it becomes a spontaneous process, the temperature should be greater than $ 1500{\,^ \circ }\,C $ according to the Ellingham diagram, you will find it when you see that it is intercepting by line of carbon monoxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




