
To determine the half-life of a radioactive element, a student plots a graph of $ln \dfrac{dN(t)}{dt}$vs $t$. Here $\dfrac {dN(t)}{dt}$ is the rate of radioactive decay at time ‘t’. If the number of radioactive nuclei of this element decreases by a factor of ‘p’ after 4.16 years, the value of ‘p’ is:
A. 9
B. 8
c. 10
D. 11

Answer
579.6k+ views
Hint: Every radio-active reaction follows first order kinetics, which means that the rate of decay of the element depends is proportional to the first power of the concentration of that element. This states that the element will decay exponentially with the time. We have well derived relations for the concentration of elements undergoing first order reactions.
Formula used:
$N = N_o e^{-\lambda t}$
Complete answer:
As the concentration of an element undergoing first order reaction varies as $N = N_o e^{-\lambda t}$ (here, N is the amount decayed after time ‘t’ from a sample of $N_o$ and $\lambda$ is a constant, called decay constant), we can easily find the rate of change of consternation as;
$N = N_o e^{-\lambda t}$
And $\dfrac{dN}{dt} = -\lambda N_o e^{-\lambda t}$
Now, taking log both sides, we get;
$ln \left[ \dfrac{dN}{dt} \right] = ln \lambda N_o - \lambda t$
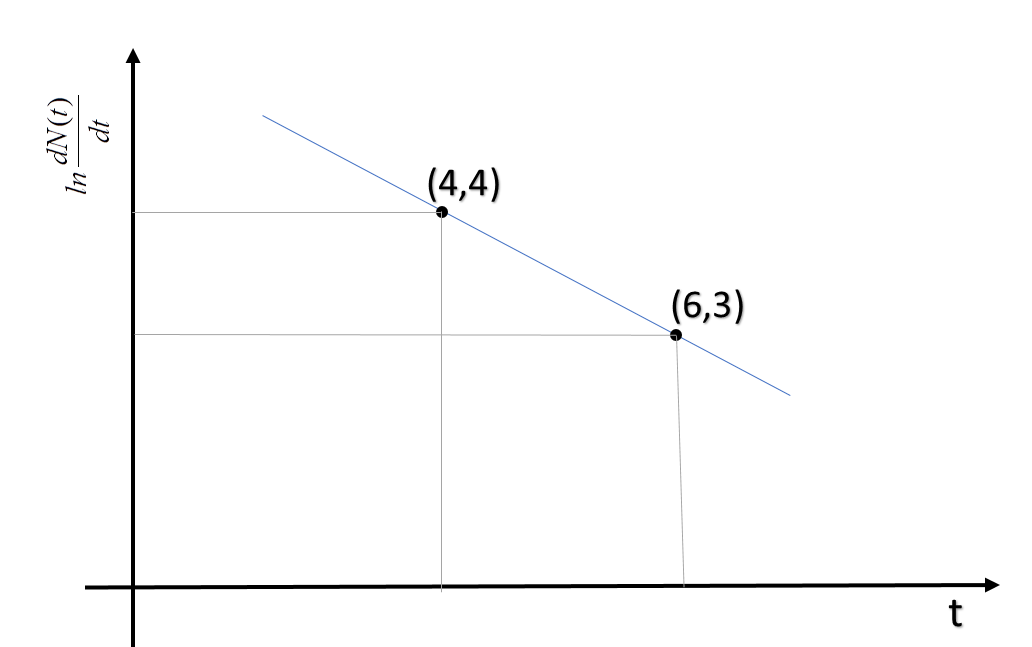
Now, from the graph, we can see, the slope of the line which is equal to $\lambda$ is $\dfrac{1}{2}$/year.
Hence, $N = N_o e^{-\lambda t}$
$N_{finally} = \dfrac{N_o}{p} = N_o e^{-\lambda t}$
$\implies p = e^{\lambda t}$
Given time = 4.16 years
So $p = e^{4.16\times \dfrac12} = e^{2.08} \approx 8$
Thus, p is roughly 8.
So, the correct answer is “Option B”.
Additional Information:
Every reaction, whether nuclear or chemical obeys a certain order of reaction. Reaction can be of zeroth order, first order, second order or higher order. The order is decided as per the behavior of each reactant. If the rate of change of concentration of reactant depends upon the power 2 of the concentration, it’s called second order. In that way, the zeroth order reaction doesn’t really depend upon the concentration of the reactant.
Note:
When-ever we have a nuclear reaction, we have to assume it first order unless stated. This is the most important reaction in chemical kinetic theory. These reactions are very useful in the laboratory in which nuclear experiments are conducted (Nuclear fission reaction experiments).
Formula used:
$N = N_o e^{-\lambda t}$
Complete answer:
As the concentration of an element undergoing first order reaction varies as $N = N_o e^{-\lambda t}$ (here, N is the amount decayed after time ‘t’ from a sample of $N_o$ and $\lambda$ is a constant, called decay constant), we can easily find the rate of change of consternation as;
$N = N_o e^{-\lambda t}$
And $\dfrac{dN}{dt} = -\lambda N_o e^{-\lambda t}$
Now, taking log both sides, we get;
$ln \left[ \dfrac{dN}{dt} \right] = ln \lambda N_o - \lambda t$
Now, from the graph, we can see, the slope of the line which is equal to $\lambda$ is $\dfrac{1}{2}$/year.
Hence, $N = N_o e^{-\lambda t}$
$N_{finally} = \dfrac{N_o}{p} = N_o e^{-\lambda t}$
$\implies p = e^{\lambda t}$
Given time = 4.16 years
So $p = e^{4.16\times \dfrac12} = e^{2.08} \approx 8$
Thus, p is roughly 8.
So, the correct answer is “Option B”.
Additional Information:
Every reaction, whether nuclear or chemical obeys a certain order of reaction. Reaction can be of zeroth order, first order, second order or higher order. The order is decided as per the behavior of each reactant. If the rate of change of concentration of reactant depends upon the power 2 of the concentration, it’s called second order. In that way, the zeroth order reaction doesn’t really depend upon the concentration of the reactant.
Note:
When-ever we have a nuclear reaction, we have to assume it first order unless stated. This is the most important reaction in chemical kinetic theory. These reactions are very useful in the laboratory in which nuclear experiments are conducted (Nuclear fission reaction experiments).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




