
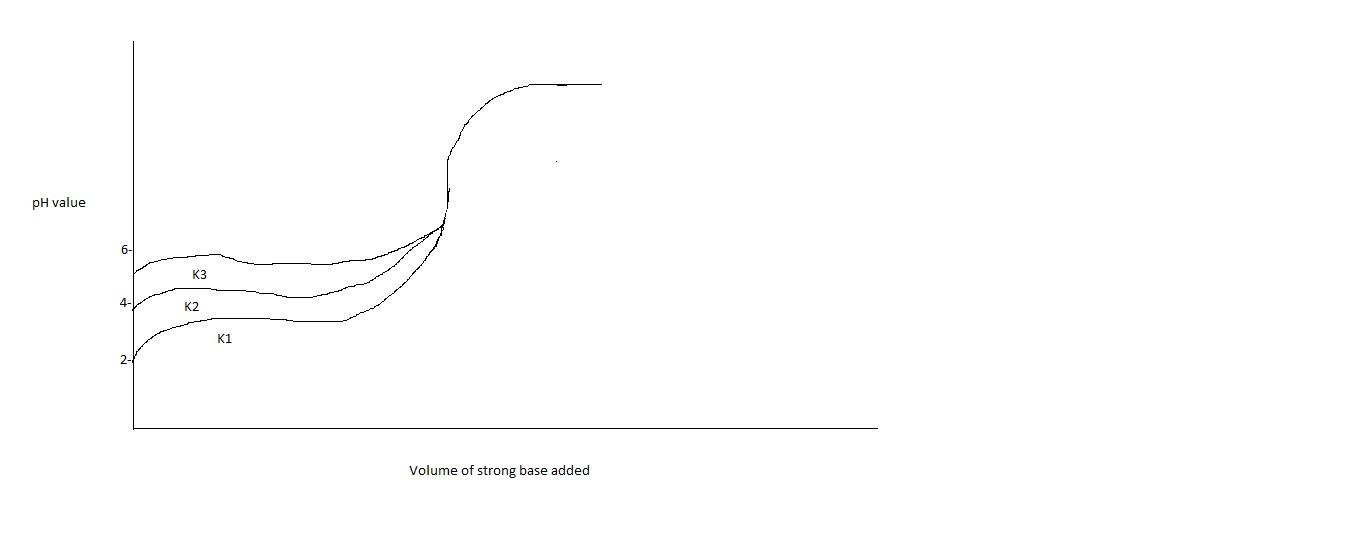
Titration curves for $0.1{\text{M}}$ solutions of three weak acids ${\text{H}}{{\text{A}}_1},{\text{H}}{{\text{A}}_2},{\text{H}}{{\text{A}}_3}$ with ionization constants ${{\text{K}}_1},{{\text{K}}_2},{{\text{K}}_3}$ respectively are plotted in the figure. Which of the following is/are true?
A. \[{{\text{K}}_2} = \dfrac{{\left( {{{\text{K}}_1} + {{\text{K}}_3}} \right)}}{2}\]
B. ${{\text{K}}_1} < {{\text{K}}_3}$
C. ${{\text{K}}_1} > {{\text{K}}_2}$
D. ${{\text{K}}_2} > {{\text{K}}_3}$

Answer
577.8k+ views
Hint:Ionization constant of an acid is the equilibrium constant for the reaction of a weak acid with water. Similarly the ionization constant of a base is the equilibrium constant for the reaction of a weak base with water. Acid strength increases with ionization constant.
Complete step by step answer:
Acids are generally defined as the hydrogen containing compounds and bases are the hydroxide containing compounds. Ionization is the process in which individual positive and negative ions are produced from a molecular compound that is dissolved in a solution. While dissociation is the process in which positive and negative ions are released from an ionic compound that is dissolved in a solution. Weak acids transfer only a small percentage of its protons to water in aqueous solution.
In the titration curve of weak acids, only a fraction of weak acid is dissociated. Before adding the base, ${\text{pH}}$ of the weak acid is greater than that of strong acid. Initially the ${\text{pH}}$ changes rapidly. At an equivalence point (the point at which neutralization is complete), the solution becomes basic. When the acid is neutralized, ${\text{pH}}$ is influenced by the addition of base in excess. Ionization constant is the equilibrium constant. When ionization constant is increased, ${\text{pH}}$ is increased.
Thus from graph, we can tell that ${{\text{K}}_1}$ has less value than ${{\text{K}}_2}$ and ${{\text{K}}_3}$. Moreover, ${{\text{K}}_2}$ is in the middle of ${{\text{K}}_1}$ and ${{\text{K}}_3}$. Therefore option A will be correct. ${{\text{K}}_2}$ will be the average of ${{\text{K}}_1}$ and ${{\text{K}}_3}$. Moreover, option B is also correct, i.e. ${{\text{K}}_1} < {{\text{K}}_3}$.
Thus options A and B are correct.
Additional information:
${\text{pH}}$ is calculated from the value of $\left[ {{{\text{H}}_3}{{\text{O}}^ + }} \right]$. It is the negative algorithm of hydronium ion concentration. In pure water hydronium concentration is the same as that of hydroxide ion concentration.
Note:
In any acid-base reaction, the equilibrium will favor the reaction that moves the proton to the stronger base. When ${\text{HCl}}$ is used, since water is stronger than ${\text{C}}{{\text{l}}^ - }$, equilibrium moves to the right. During auto-ionization, the ionization constant is called the ion-product constant.
Complete step by step answer:
Acids are generally defined as the hydrogen containing compounds and bases are the hydroxide containing compounds. Ionization is the process in which individual positive and negative ions are produced from a molecular compound that is dissolved in a solution. While dissociation is the process in which positive and negative ions are released from an ionic compound that is dissolved in a solution. Weak acids transfer only a small percentage of its protons to water in aqueous solution.
In the titration curve of weak acids, only a fraction of weak acid is dissociated. Before adding the base, ${\text{pH}}$ of the weak acid is greater than that of strong acid. Initially the ${\text{pH}}$ changes rapidly. At an equivalence point (the point at which neutralization is complete), the solution becomes basic. When the acid is neutralized, ${\text{pH}}$ is influenced by the addition of base in excess. Ionization constant is the equilibrium constant. When ionization constant is increased, ${\text{pH}}$ is increased.
Thus from graph, we can tell that ${{\text{K}}_1}$ has less value than ${{\text{K}}_2}$ and ${{\text{K}}_3}$. Moreover, ${{\text{K}}_2}$ is in the middle of ${{\text{K}}_1}$ and ${{\text{K}}_3}$. Therefore option A will be correct. ${{\text{K}}_2}$ will be the average of ${{\text{K}}_1}$ and ${{\text{K}}_3}$. Moreover, option B is also correct, i.e. ${{\text{K}}_1} < {{\text{K}}_3}$.
Thus options A and B are correct.
Additional information:
${\text{pH}}$ is calculated from the value of $\left[ {{{\text{H}}_3}{{\text{O}}^ + }} \right]$. It is the negative algorithm of hydronium ion concentration. In pure water hydronium concentration is the same as that of hydroxide ion concentration.
Note:
In any acid-base reaction, the equilibrium will favor the reaction that moves the proton to the stronger base. When ${\text{HCl}}$ is used, since water is stronger than ${\text{C}}{{\text{l}}^ - }$, equilibrium moves to the right. During auto-ionization, the ionization constant is called the ion-product constant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




