
How many times faster will nitrogen dioxide gas effuse than sulfur trioxide?
Answer
534.3k+ views
Hint: The ratio of effusion speeds of the gases can be calculated using Graham’s law of effusion. According to this law, the rate of effusion (r) of a gas is inversely proportional to the square root of the molar mass (M):
\[\text{r}\propto \dfrac{1}{\sqrt{\text{M}}}\]
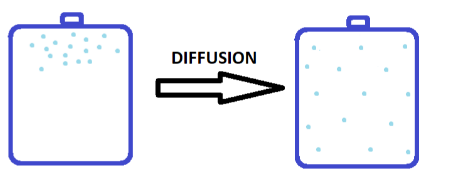
Complete step by step solution: The Kinetic theory of gases says that the gas particles are always in a constant state of motion and they move in all directions with random speeds. Due to such movement of gases, when they are inserted in a closed container, they tend to disperse throughout the container from the area of high concentration to low concentration. This process is known as diffusion.
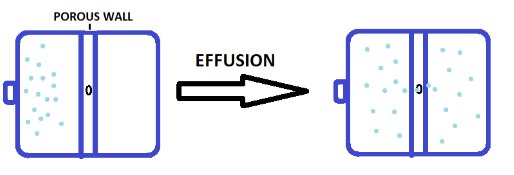
Now let us consider a situation where we have a closed container and there is a porous wall present inside. When we introduce a gas inside that container, the gas particles will tend to move from a region of higher concentration to a lower concentration. But due to the presence of small holes (or pores) on the wall, only one or few gas particles will pass through the hole at a time.
This process of escaping gas particles through a hole is known as effusion. The diameter (or the opening of the hole) should be smaller than the mean free path of the gas particles otherwise the particles cannot pass through the wall.
The mean free path is the average distance traveled by gas particles between successive collisions. So, the complete definition of the effusion is: The process in which gas passes through a small hole whose diameter is smaller than the mean free path of gas molecules is known as the effusion.
Graham’s Law of Effusion was given by Thomas Graham who studied the rate of effusion of different mixtures of gases under identical conditions. The law states that the rate of effusion of the gases is inversely proportional to the square root of their molar masses. So, the lighter gas effuses faster than heavier gas.
\[\text{r}\propto \dfrac{1}{\sqrt{\text{M}}}\]
For the mixture of two gases: $\left( \text{N}{{\text{O}}_{2}} \right)$ and $\left( \text{S}{{\text{O}}_{3}} \right)$
\[
\dfrac{{{\text{r}}_{\text{N}{{\text{O}}_{2}}}}}{{{\text{r}}_{{{\text{S}}_{2}}{{\text{O}}_{3}}}}}=\dfrac{\sqrt{{{\text{M}}_{\text{S}{{\text{O}}_{3}}}}}}{\sqrt{{{\text{M}}_{\text{N}{{\text{O}}_{2}}}}}}=\sqrt{\dfrac{{{\text{M}}_{\text{S}{{\text{O}}_{3}}}}}{{{\text{M}}_{\text{N}{{\text{O}}_{2}}}}}} \\
\because \text{ }{{\text{M}}_{\text{N}{{\text{O}}_{2}}}}=46.01\text{ g mo}{{\text{l}}^{-1}},\text{ }{{\text{M}}_{\text{S}{{\text{O}}_{3}}}}=80.07\text{ g mo}{{\text{l}}^{-1}} \\
\therefore \text{ }\dfrac{{{\text{r}}_{\text{N}{{\text{O}}_{2}}}}}{{{\text{r}}_{\text{S}{{\text{O}}_{3}}}}}=\sqrt{\dfrac{80.07}{46.01}}=1.32 \\
\Rightarrow {{\text{r}}_{\text{N}{{\text{O}}_{2}}}}=1.32\left( {{\text{r}}_{\text{S}{{\text{O}}_{3}}}} \right) \\
\]
Hence, nitrogen dioxide gas $\left( \text{N}{{\text{O}}_{2}} \right)$ will effuse 1.32 times faster than sulfur trioxide $\left( \text{S}{{\text{O}}_{3}} \right)$.
Note: Both diffusion and effusion of gases are related to their molar masses, but these are two different phenomena. Also, the rate of diffusion and the rate of effusion of a gas cannot be the same. However, their ratios can be the same.
\[\text{r}\propto \dfrac{1}{\sqrt{\text{M}}}\]
Complete step by step solution: The Kinetic theory of gases says that the gas particles are always in a constant state of motion and they move in all directions with random speeds. Due to such movement of gases, when they are inserted in a closed container, they tend to disperse throughout the container from the area of high concentration to low concentration. This process is known as diffusion.

Now let us consider a situation where we have a closed container and there is a porous wall present inside. When we introduce a gas inside that container, the gas particles will tend to move from a region of higher concentration to a lower concentration. But due to the presence of small holes (or pores) on the wall, only one or few gas particles will pass through the hole at a time.
This process of escaping gas particles through a hole is known as effusion. The diameter (or the opening of the hole) should be smaller than the mean free path of the gas particles otherwise the particles cannot pass through the wall.
The mean free path is the average distance traveled by gas particles between successive collisions. So, the complete definition of the effusion is: The process in which gas passes through a small hole whose diameter is smaller than the mean free path of gas molecules is known as the effusion.

Graham’s Law of Effusion was given by Thomas Graham who studied the rate of effusion of different mixtures of gases under identical conditions. The law states that the rate of effusion of the gases is inversely proportional to the square root of their molar masses. So, the lighter gas effuses faster than heavier gas.
\[\text{r}\propto \dfrac{1}{\sqrt{\text{M}}}\]
For the mixture of two gases: $\left( \text{N}{{\text{O}}_{2}} \right)$ and $\left( \text{S}{{\text{O}}_{3}} \right)$
\[
\dfrac{{{\text{r}}_{\text{N}{{\text{O}}_{2}}}}}{{{\text{r}}_{{{\text{S}}_{2}}{{\text{O}}_{3}}}}}=\dfrac{\sqrt{{{\text{M}}_{\text{S}{{\text{O}}_{3}}}}}}{\sqrt{{{\text{M}}_{\text{N}{{\text{O}}_{2}}}}}}=\sqrt{\dfrac{{{\text{M}}_{\text{S}{{\text{O}}_{3}}}}}{{{\text{M}}_{\text{N}{{\text{O}}_{2}}}}}} \\
\because \text{ }{{\text{M}}_{\text{N}{{\text{O}}_{2}}}}=46.01\text{ g mo}{{\text{l}}^{-1}},\text{ }{{\text{M}}_{\text{S}{{\text{O}}_{3}}}}=80.07\text{ g mo}{{\text{l}}^{-1}} \\
\therefore \text{ }\dfrac{{{\text{r}}_{\text{N}{{\text{O}}_{2}}}}}{{{\text{r}}_{\text{S}{{\text{O}}_{3}}}}}=\sqrt{\dfrac{80.07}{46.01}}=1.32 \\
\Rightarrow {{\text{r}}_{\text{N}{{\text{O}}_{2}}}}=1.32\left( {{\text{r}}_{\text{S}{{\text{O}}_{3}}}} \right) \\
\]
Hence, nitrogen dioxide gas $\left( \text{N}{{\text{O}}_{2}} \right)$ will effuse 1.32 times faster than sulfur trioxide $\left( \text{S}{{\text{O}}_{3}} \right)$.
Note: Both diffusion and effusion of gases are related to their molar masses, but these are two different phenomena. Also, the rate of diffusion and the rate of effusion of a gas cannot be the same. However, their ratios can be the same.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




