
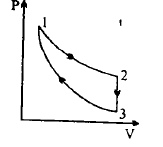
Three processes form a thermodynamic cycle as shown on $P - V$ diagram for an ideal gas. Process $1 \to 2$ takes place at a constant temperature $\left( {300K} \right)$ . Process $2 \to 3$ takes place at a constant volume. During this process $40J$ of heat leaves the system. Process $3 \to 1$ is adiabatic and temperature \[{T_3}\] is $275K$ Work done by the gas during the process $3 \to 1$ is
(a). $ - 40$ \[J\]
(b). $ - 20$ \[J\]
(c). $ + 40$ \[J\]
(d). $ + 20$ \[J\]

Answer
584.4k+ views
Hint: In this question we will come across the concept of thermodynamics. In this question we will be crossing paths with many important concepts like enthalpy, Work done and the types of thermodynamic processes. Below here we have explained each topic very properly.
Complete step-by-step answer:
Enthalpy: We know that energy change occurring during the reaction at a constant pressure and constant volume is given by internal energy change that is, heat absorbed at constant volume is equal to change in internal energy that is\[\Delta U = {q_v}\]. As atmospheric pressure is constant, therefore, such reactions may involve change in volume. It is a sum of internal energy and pressure-volume energy of the system at a particular temperature and pressure.
Work done: Is also a mode of transference of energy between system and surroundings. Work done by the system on the surrounding is given by P\[\Delta \]V.
Thermodynamic Process: If a system certain temperature change occurs the system that will be formed is regarded as a state system. The process of changing state is named as thermodynamic processes. These are of following types:
Isothermal Process: Temperature is the same.
Adiabatic Process: No transfer of heat from system to surrounding and vice versa.
Isobaric Processes: pressure is same
Isochoric Process: Volume is same
Cyclic Processes: same initial and final state
In the processes \[1 \to 2,\Delta {Q_{1 \to 2}} = \Delta {W_{1 \to 2}}\]
in the process \[2 \to 3,\Delta {W_{2 \to 3}} = 0\]
in the process \[3 \to 1,\Delta {Q_{3 \to 1}} = 0\]
by figure we can say that the process is cyclic,\[\Delta U = 0\]
So\[\Delta Q = \Delta W\]
\[\$
\Rightarrow \Delta {Q_{1 \to 2}} + \Delta {Q_{2 \to 3}} + \Delta {Q_{3 \to 1}} = \Delta {W_{1 \to 2}} + \Delta {W_{2 \to 3}} + \Delta {W_{3 \to 1}} \\
\Rightarrow \Delta {Q_{2 \to 3}} = \Delta {W_{3 \to 1}} = - 40J \\
$ \]
So, we concluded that option (A) is correct.
Hence, the correct answer is option A.
Note: In this question we have learned about enthalpy, thermodynamic process and its type and about work done. Change in enthalpy is measured by calorimeter and the process is called calorimeter. This information we have learned in this question will help us in future, in solving the questions of this kind.
Complete step-by-step answer:
Enthalpy: We know that energy change occurring during the reaction at a constant pressure and constant volume is given by internal energy change that is, heat absorbed at constant volume is equal to change in internal energy that is\[\Delta U = {q_v}\]. As atmospheric pressure is constant, therefore, such reactions may involve change in volume. It is a sum of internal energy and pressure-volume energy of the system at a particular temperature and pressure.
Work done: Is also a mode of transference of energy between system and surroundings. Work done by the system on the surrounding is given by P\[\Delta \]V.
Thermodynamic Process: If a system certain temperature change occurs the system that will be formed is regarded as a state system. The process of changing state is named as thermodynamic processes. These are of following types:
Isothermal Process: Temperature is the same.
Adiabatic Process: No transfer of heat from system to surrounding and vice versa.
Isobaric Processes: pressure is same
Isochoric Process: Volume is same
Cyclic Processes: same initial and final state
In the processes \[1 \to 2,\Delta {Q_{1 \to 2}} = \Delta {W_{1 \to 2}}\]
in the process \[2 \to 3,\Delta {W_{2 \to 3}} = 0\]
in the process \[3 \to 1,\Delta {Q_{3 \to 1}} = 0\]
by figure we can say that the process is cyclic,\[\Delta U = 0\]
So\[\Delta Q = \Delta W\]
\[\$
\Rightarrow \Delta {Q_{1 \to 2}} + \Delta {Q_{2 \to 3}} + \Delta {Q_{3 \to 1}} = \Delta {W_{1 \to 2}} + \Delta {W_{2 \to 3}} + \Delta {W_{3 \to 1}} \\
\Rightarrow \Delta {Q_{2 \to 3}} = \Delta {W_{3 \to 1}} = - 40J \\
$ \]
So, we concluded that option (A) is correct.
Hence, the correct answer is option A.
Note: In this question we have learned about enthalpy, thermodynamic process and its type and about work done. Change in enthalpy is measured by calorimeter and the process is called calorimeter. This information we have learned in this question will help us in future, in solving the questions of this kind.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




