
This method of separation is called:
A-Fractionation
B-Condensation
C-Evaporation
D-Distillation

Answer
564.9k+ views
Hint: Various methods are used for purification of substances. If the two components of a mixture have sufficient difference in their boiling points, they can be separated by boiling first and then condensing the component which boiled first.
Complete answer:
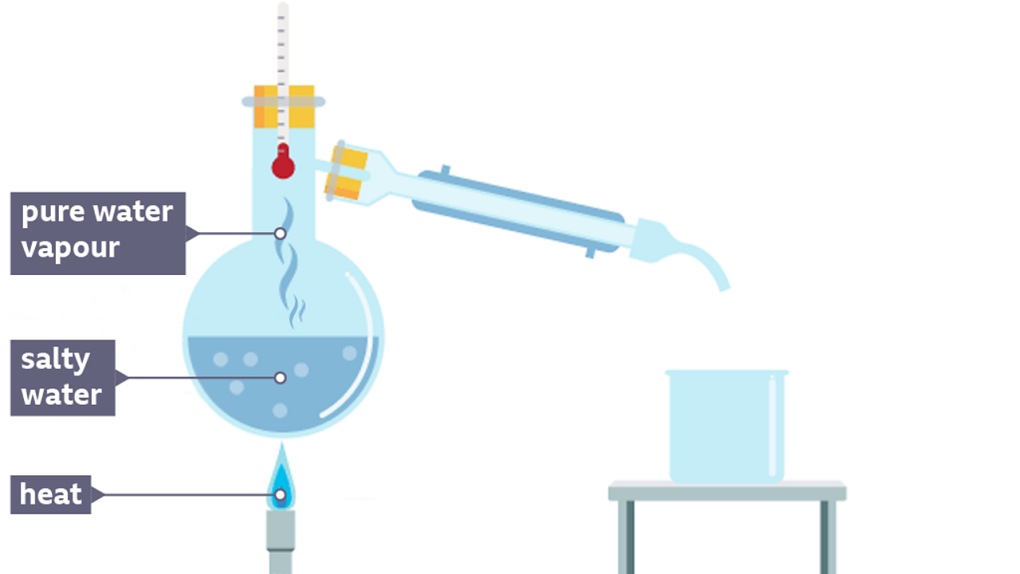
We have often heard the term distillation. Simple distillation is the process of separating components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points. It is a separation technique generally used either to concentrate a given substance or to separate it from a mixture.
So, in the above given set up as the water is heated in the flask, it will start forming vapours. But the salt will remain behind. As the vapours will move through the bent tube, it will be cooled by the cold-water present in the insulation layer around it which will convert it back to water. This pure water gets collected in the beaker.
Hence, option D is correct.
Additional information:
In simple distillation liquid mixture is heated till its boiling point and the resulting vapours are condensed immediately. The resulting liquid is called a distillate and its purity is measured by Roult’s law. This method is effective only when the difference between the boiling points is sufficiently large.
Note:
The process of distillation exploits the difference in the boiling points of the components in the liquid mixture by forcing one of them into a gaseous state.
Complete answer:
We have often heard the term distillation. Simple distillation is the process of separating components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points. It is a separation technique generally used either to concentrate a given substance or to separate it from a mixture.
So, in the above given set up as the water is heated in the flask, it will start forming vapours. But the salt will remain behind. As the vapours will move through the bent tube, it will be cooled by the cold-water present in the insulation layer around it which will convert it back to water. This pure water gets collected in the beaker.
Hence, option D is correct.
Additional information:
In simple distillation liquid mixture is heated till its boiling point and the resulting vapours are condensed immediately. The resulting liquid is called a distillate and its purity is measured by Roult’s law. This method is effective only when the difference between the boiling points is sufficiently large.
Note:
The process of distillation exploits the difference in the boiling points of the components in the liquid mixture by forcing one of them into a gaseous state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




