
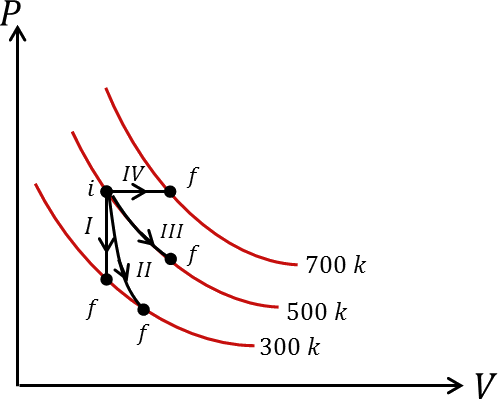
Thermodynamic processes are indicated in the following diagram.
Match the following:
Column-I Column-II P. Process I a. Adiabatic Q. Process II b. Isobaric R. Process III c. Isochoric S. Process IV d. Isothermal
A. P-d, Q-B, R-a, S-c
B. P-a, Q-c, R-d, S-b
C. P-c, Q-a, R-d, S-b
D. P-c, Q-d, R-b, S-a
| Column-I | Column-II |
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |

Answer
540k+ views
Hint: Adiabatic process is a process in which there is no transfer of heat between the surrounding and the system. Isobaric process is a process in which the pressure remains constant. Isochoric process is a process in which the volume remains constant. Isothermal process is a process in which the temperature of the system remains constant.
Complete answer:
In the given diagram, we can understand the process I depicted constant volume which means it is an Isochoric Process.
Process III shows constant temperature which means it is an Isothermal Process.
Process IV shows constant pressure which means it is an Isobaric Process.
The only option which matches the above explanation is option C which tells that the process II is an Adiabatic Process.
Thus, Process I is Isochoric, Process II is Adiabatic, Process III is Isothermal and Process IV is Isobaric.
So, the correct answer is option C i.e. P-c, Q-a, R-d, S-b.
Note:
To answer these types of questions, students must have the concepts and applications of thermodynamics clear. Similarly, other curves such as Pressure vs Temperature which depicts variation in pressure and temperature, and Volume vs Temperature which depicts the variation in volume and temperature can also be asked.
Complete answer:
In the given diagram, we can understand the process I depicted constant volume which means it is an Isochoric Process.
Process III shows constant temperature which means it is an Isothermal Process.
Process IV shows constant pressure which means it is an Isobaric Process.
The only option which matches the above explanation is option C which tells that the process II is an Adiabatic Process.
Thus, Process I is Isochoric, Process II is Adiabatic, Process III is Isothermal and Process IV is Isobaric.
So, the correct answer is option C i.e. P-c, Q-a, R-d, S-b.
Note:
To answer these types of questions, students must have the concepts and applications of thermodynamics clear. Similarly, other curves such as Pressure vs Temperature which depicts variation in pressure and temperature, and Volume vs Temperature which depicts the variation in volume and temperature can also be asked.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




