
There are three common ways to convert an alkene into alcohol:
(A) acid catalyzed hydration
(B) hydroboration-oxidation
(C) oxymercuration-demercuration.
Which of the following statements about these reactions is incorrect?
(a) (1) $C{{l}_{2}}/hv$ followed by $KOH$ (2) $Br/CC{{l}_{4}}$ followed by $KOH$ (b) (1) $Br/heat$ followed by $KOH$ (2) $NBS/DMSO/{{H}_{2}}O$ followed by $KOH$ (c) (1) $C{{l}_{2}}/hv$ followed by $NaOH$ (2) $HBr$ followed by $KOH$ (d) (1) $B{{r}_{2}}/hv$ followed by $NaOH$ (2) $Os{{O}_{4}}/NaHS{{O}_{3}}$ followed by $HBr$
| (a) | (1) $C{{l}_{2}}/hv$ followed by $KOH$ | (2) $Br/CC{{l}_{4}}$ followed by $KOH$ |
| (b) | (1) $Br/heat$ followed by $KOH$ | (2) $NBS/DMSO/{{H}_{2}}O$ followed by $KOH$ |
| (c) | (1) $C{{l}_{2}}/hv$ followed by $NaOH$ | (2) $HBr$ followed by $KOH$ |
| (d) | (1) $B{{r}_{2}}/hv$ followed by $NaOH$ | (2) $Os{{O}_{4}}/NaHS{{O}_{3}}$ followed by $HBr$ |
Answer
559.2k+ views
Hint: First you should all the reactions of the alkenes properly i.e. its method of preparation and its chemical reactions and then, you can easily identify that reactant from the given options which will not give alcohols when alkenes react with it.
Complete answer:
First of let’s discuss the reactions used to convert the alkene into alcohol.
(A) acid catalyzed hydration:- In this reaction, alkene is made to react with the water which results in the formation of the alcohol and unsaturated compound changes to the saturated compound. The reaction occurs as-
$C{{H}_{2}}=C{{H}_{2}}+H-OH\to H-C{{H}_{2}}-C{{H}_{2}}-OH$
(B) hydroboration-oxidation:- In this reaction, alkene is made to react with the borane resulting in the formation of the alcohol. The general reaction is supposed to occurs as;
$R-C=C-R+B{{H}_{3}}\to R-C-C-OH$
(C) oxymercuration-demercuration:- In this reaction, alkene is made to react with the mercury acetate along with the water and an intermediate is formed which on reaction with the $NaB{{H}_{4}}$ gives the alcohols. The reaction occurs as;
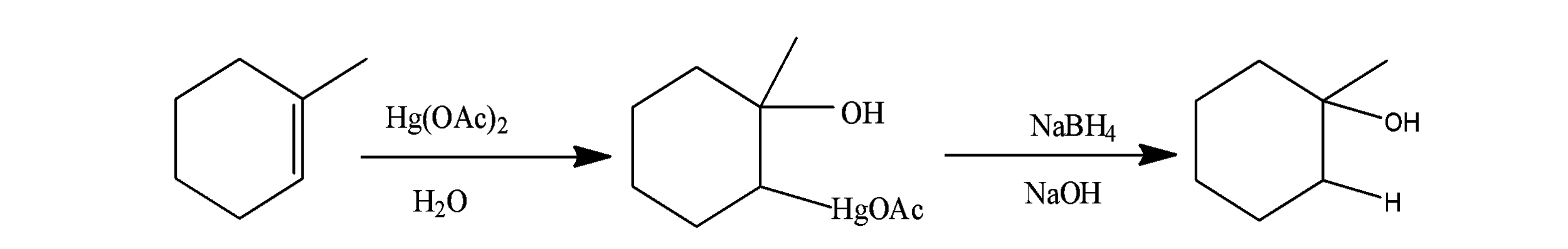
Now considering the statement-
Out of all the options, given above , option (A) is incorrect. It is so because the alcoholic KOH is used in the preparation of the alkene and it undergoes dehalogenation reaction and it doesn’t form the alcohols rather forms alkenes again.
While rest all other reactants when made to undergo reaction with the alkenes form the alcohols.
Hence, option (A) is correct.
Note:
Alkenes are the unsaturated organic compounds which contain the carbon -carbon double bond in their compounds whereas on the other hand alcohols are the hydroxy derivatives ($-OH$) of aliphatic hydrocarbons.
Complete answer:
First of let’s discuss the reactions used to convert the alkene into alcohol.
(A) acid catalyzed hydration:- In this reaction, alkene is made to react with the water which results in the formation of the alcohol and unsaturated compound changes to the saturated compound. The reaction occurs as-
$C{{H}_{2}}=C{{H}_{2}}+H-OH\to H-C{{H}_{2}}-C{{H}_{2}}-OH$
(B) hydroboration-oxidation:- In this reaction, alkene is made to react with the borane resulting in the formation of the alcohol. The general reaction is supposed to occurs as;
$R-C=C-R+B{{H}_{3}}\to R-C-C-OH$
(C) oxymercuration-demercuration:- In this reaction, alkene is made to react with the mercury acetate along with the water and an intermediate is formed which on reaction with the $NaB{{H}_{4}}$ gives the alcohols. The reaction occurs as;

Now considering the statement-
Out of all the options, given above , option (A) is incorrect. It is so because the alcoholic KOH is used in the preparation of the alkene and it undergoes dehalogenation reaction and it doesn’t form the alcohols rather forms alkenes again.
While rest all other reactants when made to undergo reaction with the alkenes form the alcohols.
Hence, option (A) is correct.
Note:
Alkenes are the unsaturated organic compounds which contain the carbon -carbon double bond in their compounds whereas on the other hand alcohols are the hydroxy derivatives ($-OH$) of aliphatic hydrocarbons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




