
The yield of acetanilide in the reaction ($100\% $ conversion) of $2$ moles of aniline with $1$ mole of acetic anhydride is:
A. $135g$
B. $135g$
C. $67.5g$
D. $177g$
Answer
546.9k+ views
Hint: Write the reaction of conversion of aniline to acetanilide, as given ($100\% $ conversion) thus it means that no loss of compound is gone during the reaction and yield is full. See the moles of compound reacting initially and then after a certain time. Yield can be defined as the mass divided by molar mass; this is the same as the formula of moles.
Complete step-by-step answer:
We have the chemical reaction between aniline and acetic anhydride after that we got acetanilide. If we see the reaction it will be written as this,
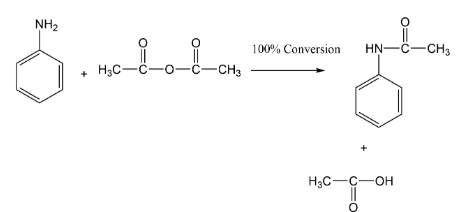
In the reaction it is given as $100\% $ conversion it means that reactants are fully converted into products with no wastage. Initially we have $2$ moles of aniline and $1$ mole of acetic anhydride as reactant side while initially the formation of product is nothing therefore product can be taken as $0\,moles$ .
As reaction proceeds towards the forward step the rate of decrease of reactants starts and formation of products starts. We are seeing the stoichiometry of the reaction. We can say that in the above reaction the limiting agent is acetic anhydride because of which the yield of the product is limited. Here, as reaction proceeds we get one mole of aniline left while on the product side the acetanilide formed and acetic acid is both one mole.
Acetanilide mass ${C_8}{H_9}NO = \,8 \times (12) + 9 \times (1) + 14 + 16\, = \,135\,gm$
The amount of acetanilide formed is one mole, which can be defined as given mass divided by molar mass. As molar mass of acetanilide is $135\,g$ therefore the yield of it in this reaction is this.
Option B is correct.
Note: For finding the yield of any reaction you have to use the information regarding moles used of reactants and moles of products formed. In some cases stoichiometry differs hence in those cases we have to see the moles formed. The yield is said to be good if it comes out greater than $80\% $ The loss of the difference is due to the errors in method or personal errors.
Complete step-by-step answer:
We have the chemical reaction between aniline and acetic anhydride after that we got acetanilide. If we see the reaction it will be written as this,

In the reaction it is given as $100\% $ conversion it means that reactants are fully converted into products with no wastage. Initially we have $2$ moles of aniline and $1$ mole of acetic anhydride as reactant side while initially the formation of product is nothing therefore product can be taken as $0\,moles$ .
As reaction proceeds towards the forward step the rate of decrease of reactants starts and formation of products starts. We are seeing the stoichiometry of the reaction. We can say that in the above reaction the limiting agent is acetic anhydride because of which the yield of the product is limited. Here, as reaction proceeds we get one mole of aniline left while on the product side the acetanilide formed and acetic acid is both one mole.
Acetanilide mass ${C_8}{H_9}NO = \,8 \times (12) + 9 \times (1) + 14 + 16\, = \,135\,gm$
The amount of acetanilide formed is one mole, which can be defined as given mass divided by molar mass. As molar mass of acetanilide is $135\,g$ therefore the yield of it in this reaction is this.
Option B is correct.
Note: For finding the yield of any reaction you have to use the information regarding moles used of reactants and moles of products formed. In some cases stoichiometry differs hence in those cases we have to see the moles formed. The yield is said to be good if it comes out greater than $80\% $ The loss of the difference is due to the errors in method or personal errors.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




