
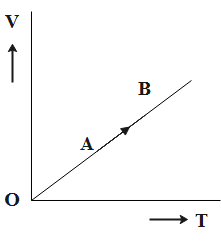
The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state A to state B, is?
\[A.\,\dfrac{1}{3}\]
\[B.\,\dfrac{2}{5}\]
\[C.\,\dfrac{2}{7}\]
\[D.\,\dfrac{2}{3}\]

Answer
574.5k+ views
Hint: This problem is a direct question. We will make use of the specific heat at a constant pressure of monatomic gas and substitute the same value in the equation of heat absorbed. Then we will divide this equation by the equation of the work done in the case of the isobaric process. Thus, we will get the required ratio.
Complete step-by-step solution
From given problem, we have a monatomic gas, so, we have,
\[{{C}_{P}}=\dfrac{5}{2}R\]
Or we can compute the specific heat at constant pressure as follows.
\[\begin{align}
& {{C}_{P}}=\dfrac{3}{2}R+R \\
& {{C}_{P}}=\dfrac{5}{2}R \\
\end{align}\]
Now, we will consider the heat absorbed.
\[dQ=n{{C}_{p}}dT\]
Substitute the equation of the specific heat at constant pressure expression in the above equation.
\[dQ=n\left( \dfrac{5}{2}R \right)dT\]
For an isobaric process, the work done is given as follows.
\[\begin{align}
& dW=P\times dV \\
& dW=nRdT \\
\end{align}\]
The ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state A to state B, is
\[\begin{align}
& \text{Ratio}=\dfrac{dW}{dQ} \\
& \text{Ratio}=\dfrac{nRdT}{n\left( \dfrac{5}{2}R \right)dT} \\
\end{align}\]
\[\therefore \] The ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state A to state B is give as follows,
\[\text{Ratio}=\dfrac{2}{5}\]
As the ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state A to state B be \[\dfrac{2}{5}\], thus, the option (B) is correct.
Note: If we know the formula for computing the excess pressure inside a soap bubble, then, we can solve this problem, otherwise, we need to find the formula first. The derivation for the excess pressure inside a soap bubble is discussed above to make the student know how we obtain the formula for computing the excess pressure inside a soap bubble.
Complete step-by-step solution
From given problem, we have a monatomic gas, so, we have,
\[{{C}_{P}}=\dfrac{5}{2}R\]
Or we can compute the specific heat at constant pressure as follows.
\[\begin{align}
& {{C}_{P}}=\dfrac{3}{2}R+R \\
& {{C}_{P}}=\dfrac{5}{2}R \\
\end{align}\]
Now, we will consider the heat absorbed.
\[dQ=n{{C}_{p}}dT\]
Substitute the equation of the specific heat at constant pressure expression in the above equation.
\[dQ=n\left( \dfrac{5}{2}R \right)dT\]
For an isobaric process, the work done is given as follows.
\[\begin{align}
& dW=P\times dV \\
& dW=nRdT \\
\end{align}\]
The ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state A to state B, is
\[\begin{align}
& \text{Ratio}=\dfrac{dW}{dQ} \\
& \text{Ratio}=\dfrac{nRdT}{n\left( \dfrac{5}{2}R \right)dT} \\
\end{align}\]
\[\therefore \] The ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state A to state B is give as follows,
\[\text{Ratio}=\dfrac{2}{5}\]
As the ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state A to state B be \[\dfrac{2}{5}\], thus, the option (B) is correct.
Note: If we know the formula for computing the excess pressure inside a soap bubble, then, we can solve this problem, otherwise, we need to find the formula first. The derivation for the excess pressure inside a soap bubble is discussed above to make the student know how we obtain the formula for computing the excess pressure inside a soap bubble.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




