
The types of hybrid orbitals of nitrogen in \[NO_{2}^{+}\], \[NO_{3}^{-}\] and \[NH_{4}^{+}\] respectively are expected to be:
A. \[sp,\text{ }s{{p}^{3}}\text{ }and\text{ }s{{p}^{2}}\]
B. \[sp,\text{ }s{{p}^{2}}\text{ }and\text{ }s{{p}^{3}}\]
C. \[s{{p}^{2}}\text{, }sp\text{ }and\text{ }s{{p}^{3}}\]
D. \[s{{p}^{2}},\text{ }s{{p}^{3}}\text{ }and\text{ }sp\]
Answer
588.6k+ views
Hint: To find the types of hybrid orbitals involved in hybridization there is a formula. It is as follows.
\[H=\dfrac{1}{2}[VC+A]\]
Where H is the number of orbitals involved in hybridization
V is the number of valence electrons of central atom
M is the number of monovalent atoms attached to central atom
C = charge on the cation
A = charge on the anion
Complete step by step answer:
- The given molecules are\[NO_{2}^{+}\], \[NO_{3}^{-}\] and \[NH_{4}^{+}\].
- The structure of \[NO_{2}^{+}\] is as follows.
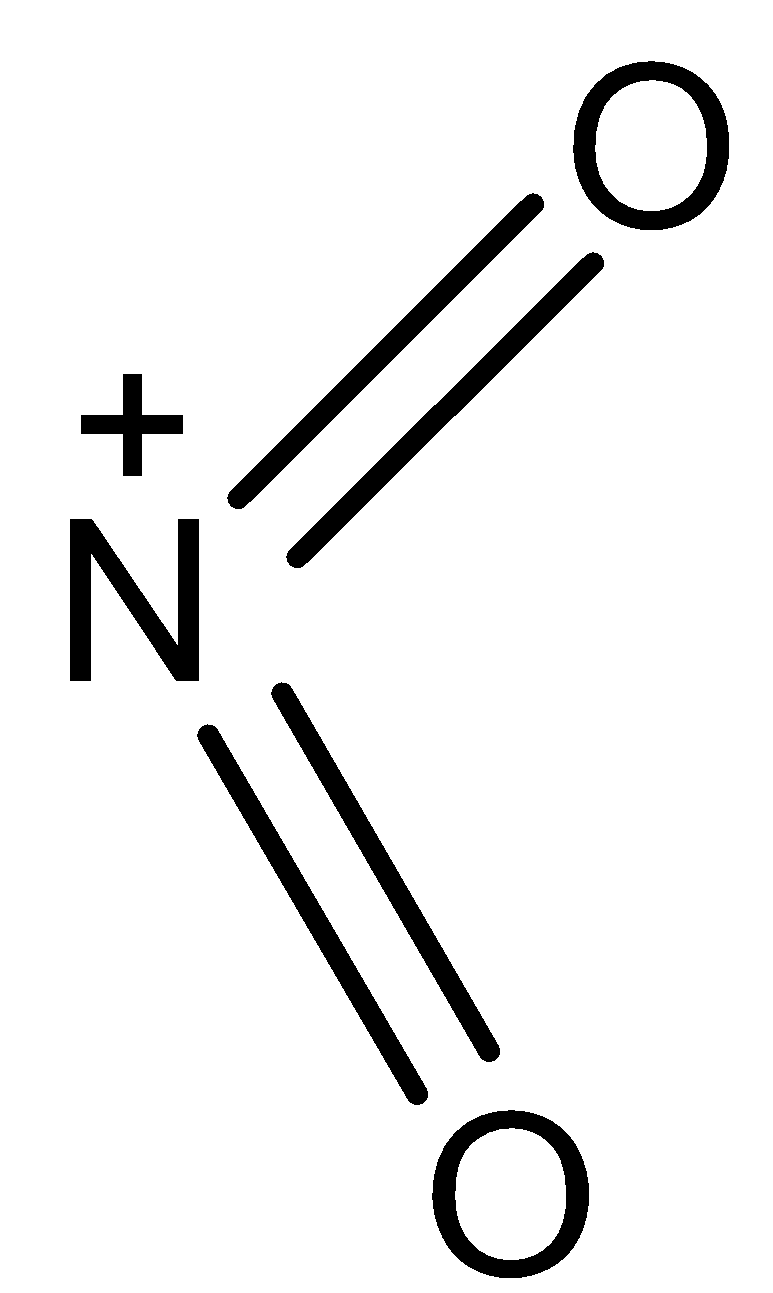
-From the above structure we can say that
V= number of valence electrons of central atom = 5
M = number of monovalent atoms attached to central atom = 0
C = charge on the cation = 1
A = charge on the anion = 0
- Therefore
\[\begin{align}
& H=\dfrac{1}{2}[V+M-C+A] \\
& H=\dfrac{1}{2}[5+0-1+0] \\
& H=2 \\
\end{align}\]
- Number of hybrid orbital is equal to two means the hybridization is \[sp\].
- The structure of \[NO_{3}^{-}\] is as follows.
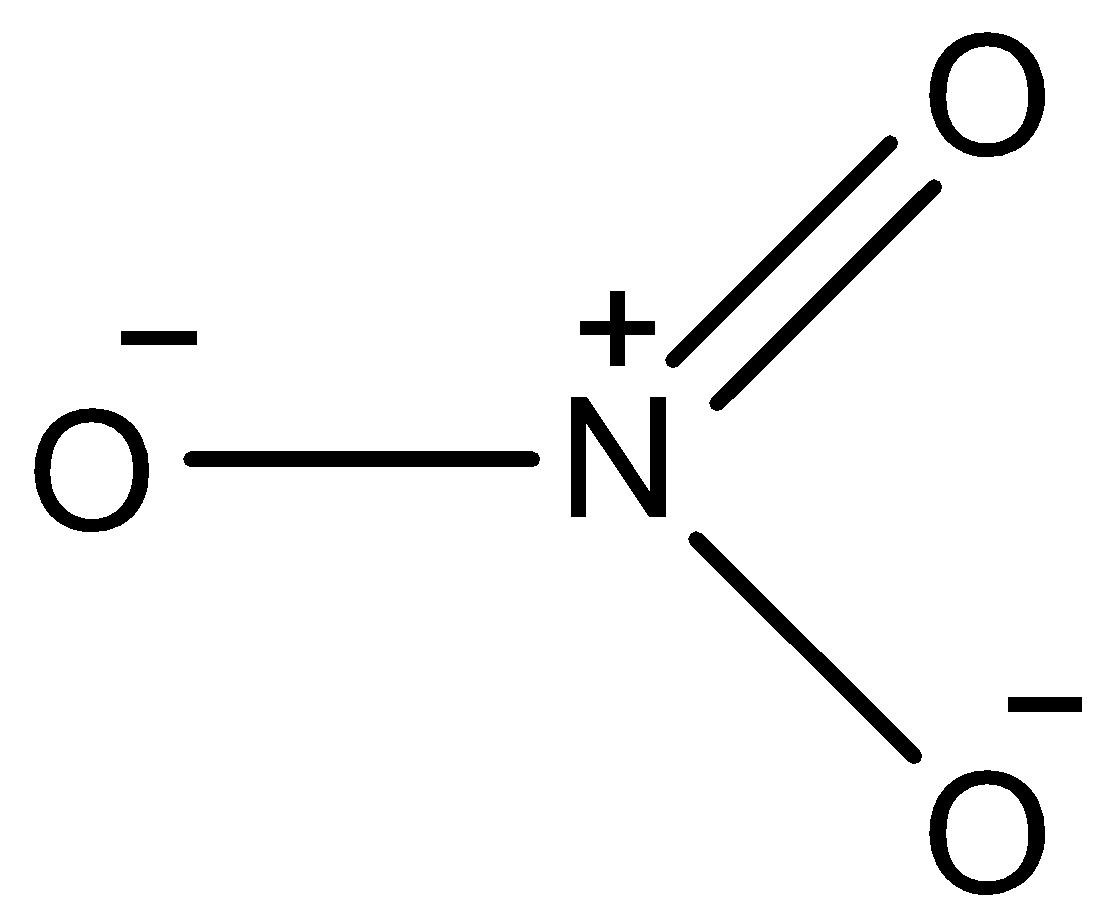
-From the above structure we can say that
V= number of valence electrons of central atom = 5
M = number of monovalent atoms attached to central atom = 0
C = charge on the cation = 0
A = charge on the anion = 1
- Therefore
\[\begin{align}
& H=\dfrac{1}{2}[V+M-C+A] \\
& H=\dfrac{1}{2}[5+0-0+1] \\
& H=3 \\
\end{align}\]
-Number of hybrid orbital is equal to three means the hybridization is \[s{{p}^{2}}\].
-The structure of \[NH_{4}^{+}\] is as follows.
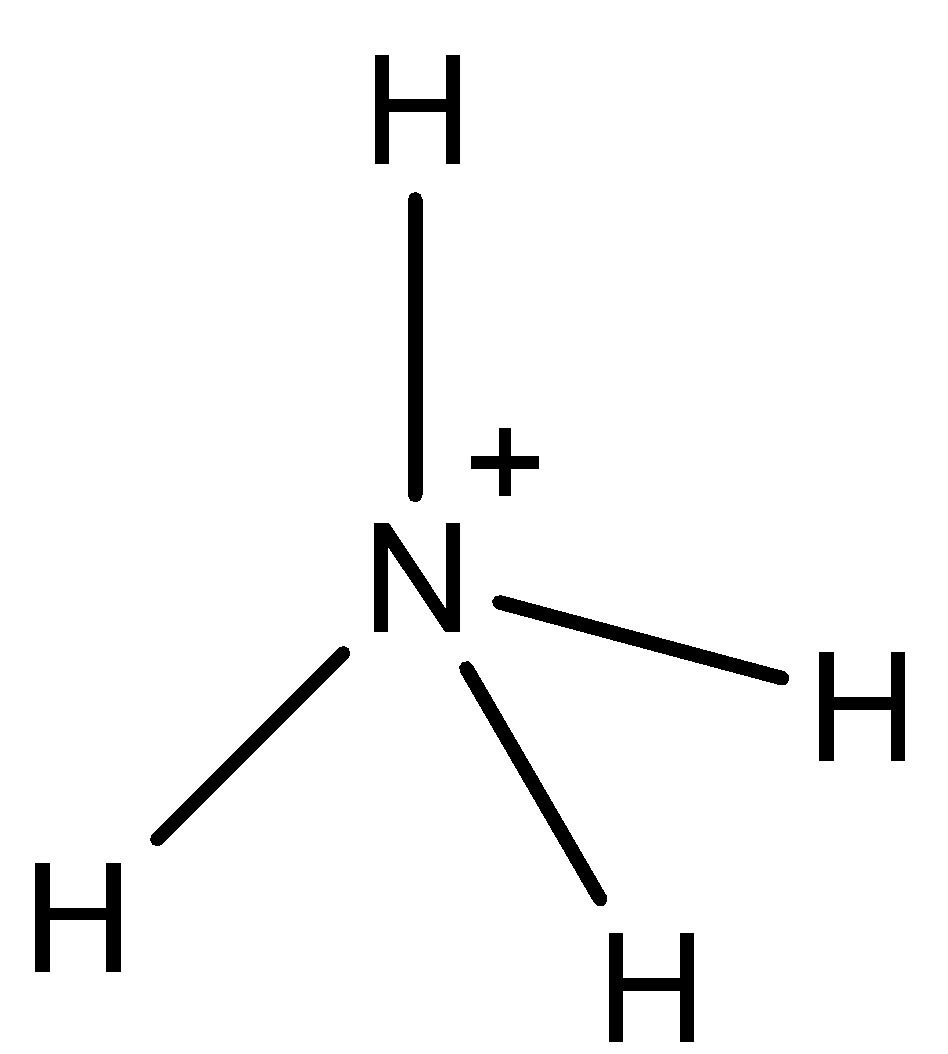
-From the above structure we can say that
V= number of valence electrons of central atom = 5
M = number of monovalent atoms attached to central atom = 4
C = charge on the cation = 1
A = charge on the anion = 0
- Therefore
\[\begin{align}
& H=\dfrac{1}{2}[V+M-C+A] \\
& H=\dfrac{1}{2}[5+4-1+0] \\
& H=4 \\
\end{align}\]
-Number of hybrid orbital is equal to four means the hybridization is \[s{{p}^{3}}\].
-The hybrid orbitals of nitrogen in \[NO_{2}^{+}\], \[NO_{3}^{-}\] and \[NH_{4}^{+}\] are \[sp,\text{ }s{{p}^{2}}\text{ }and\text{ }s{{p}^{3}}\]respectively.
So, the correct answer is “Option B”.
Note: If the number of orbitals involved in hybridization are 4 then the hybridization of the central atom is \[s{{p}^{3}}d\]. If the number of orbitals involved in hybridization are 5 then the hybridization of the central atom is \[s{{p}^{3}}{{d}^{2}}\]. If the number of orbitals involved in hybridization are 6 then the hybridization of the central atom is \[s{{p}^{3}}{{d}^{3}}\].
\[H=\dfrac{1}{2}[VC+A]\]
Where H is the number of orbitals involved in hybridization
V is the number of valence electrons of central atom
M is the number of monovalent atoms attached to central atom
C = charge on the cation
A = charge on the anion
Complete step by step answer:
- The given molecules are\[NO_{2}^{+}\], \[NO_{3}^{-}\] and \[NH_{4}^{+}\].
- The structure of \[NO_{2}^{+}\] is as follows.

-From the above structure we can say that
V= number of valence electrons of central atom = 5
M = number of monovalent atoms attached to central atom = 0
C = charge on the cation = 1
A = charge on the anion = 0
- Therefore
\[\begin{align}
& H=\dfrac{1}{2}[V+M-C+A] \\
& H=\dfrac{1}{2}[5+0-1+0] \\
& H=2 \\
\end{align}\]
- Number of hybrid orbital is equal to two means the hybridization is \[sp\].
- The structure of \[NO_{3}^{-}\] is as follows.

-From the above structure we can say that
V= number of valence electrons of central atom = 5
M = number of monovalent atoms attached to central atom = 0
C = charge on the cation = 0
A = charge on the anion = 1
- Therefore
\[\begin{align}
& H=\dfrac{1}{2}[V+M-C+A] \\
& H=\dfrac{1}{2}[5+0-0+1] \\
& H=3 \\
\end{align}\]
-Number of hybrid orbital is equal to three means the hybridization is \[s{{p}^{2}}\].
-The structure of \[NH_{4}^{+}\] is as follows.

-From the above structure we can say that
V= number of valence electrons of central atom = 5
M = number of monovalent atoms attached to central atom = 4
C = charge on the cation = 1
A = charge on the anion = 0
- Therefore
\[\begin{align}
& H=\dfrac{1}{2}[V+M-C+A] \\
& H=\dfrac{1}{2}[5+4-1+0] \\
& H=4 \\
\end{align}\]
-Number of hybrid orbital is equal to four means the hybridization is \[s{{p}^{3}}\].
-The hybrid orbitals of nitrogen in \[NO_{2}^{+}\], \[NO_{3}^{-}\] and \[NH_{4}^{+}\] are \[sp,\text{ }s{{p}^{2}}\text{ }and\text{ }s{{p}^{3}}\]respectively.
So, the correct answer is “Option B”.
Note: If the number of orbitals involved in hybridization are 4 then the hybridization of the central atom is \[s{{p}^{3}}d\]. If the number of orbitals involved in hybridization are 5 then the hybridization of the central atom is \[s{{p}^{3}}{{d}^{2}}\]. If the number of orbitals involved in hybridization are 6 then the hybridization of the central atom is \[s{{p}^{3}}{{d}^{3}}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




