
The type of bonds present in \[CuS{O_4}.5{H_2}O\] are only:
(a) Electrovalent and covalent
(b) Electrovalent and coordinate covalent
(c) Electrovalent, covalent and coordinate covalent
(d) covalent and coordinate covalent
Answer
602.7k+ views
We know that elements and compounds form bonds among themselves to stabilize. Before solving this question it is understood that chemical compounds are reliant on the strength of the chemical bonds between its constituents; the stronger the bonding between the constituents, the more stable the resulting compound would be.
Complete step by step solution:
We must first learn about the different kinds of bonding provided as an option in the question here.
i) Ionic bonding involves a transfer of an electron, so one atom gains an electron while one atom loses an electron.
ii) A covalent bond involves the sharing of electrons between two atoms. The pair of shared electrons forms a new orbit that extends around the nuclei of both atoms, producing a molecule.
iii) A covalent chemical bond between two atoms that is produced when one atom shares a pair of electrons with another atom lacking such a pair. Also called coordinate covalent bond.
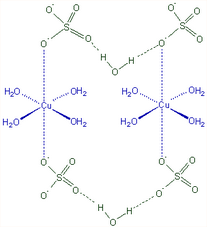
In the picture, we see that \[CuS{O_4}\] is an ionic compound. But when it is hydrated, it forms hydrogen bonding with one of the water molecules and coordinate bonds with the other 4 water molecules hence we can say it has covalent bonding in \[SO_4^{2 - }\].
The bonding in the compound is as follows:
Electrovalent (Ionic interaction between \[C{u^{2 + }}\] & \[SO_4^{2 - }\] )
Covalent (with \[SO_4^{2 - }\])
Coordinate covalent (\[{H_2}O\] molecules to \[C{u^{2 + }}\] )
Hence, we can conclude that \[CuS{O_4}.5{H_2}O\] has all 3 kinds of bonding.
Thus, Option (C) is the correct answer.
Note: We can easily remove the water of crystallization from hydrated copper (II) sulfate by heating. Condensing the vapor produced in a second test-tube collects the water, we observe the white anhydrous copper (II) sulfate. If it is rehydrated the blue color returns.
Complete step by step solution:
We must first learn about the different kinds of bonding provided as an option in the question here.
i) Ionic bonding involves a transfer of an electron, so one atom gains an electron while one atom loses an electron.
ii) A covalent bond involves the sharing of electrons between two atoms. The pair of shared electrons forms a new orbit that extends around the nuclei of both atoms, producing a molecule.
iii) A covalent chemical bond between two atoms that is produced when one atom shares a pair of electrons with another atom lacking such a pair. Also called coordinate covalent bond.

In the picture, we see that \[CuS{O_4}\] is an ionic compound. But when it is hydrated, it forms hydrogen bonding with one of the water molecules and coordinate bonds with the other 4 water molecules hence we can say it has covalent bonding in \[SO_4^{2 - }\].
The bonding in the compound is as follows:
Electrovalent (Ionic interaction between \[C{u^{2 + }}\] & \[SO_4^{2 - }\] )
Covalent (with \[SO_4^{2 - }\])
Coordinate covalent (\[{H_2}O\] molecules to \[C{u^{2 + }}\] )
Hence, we can conclude that \[CuS{O_4}.5{H_2}O\] has all 3 kinds of bonding.
Thus, Option (C) is the correct answer.
Note: We can easily remove the water of crystallization from hydrated copper (II) sulfate by heating. Condensing the vapor produced in a second test-tube collects the water, we observe the white anhydrous copper (II) sulfate. If it is rehydrated the blue color returns.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




