
The two sulphur atoms in $N{a_2}{S_2}{O_3}$ have:
(A) $ - 2$ and $ + 4$ oxidation states
(B) $ + 4$ and $ + 6$ oxidation states
(C) $ + 6$ and $ - 2$ oxidation states
(D) $0$ and $ + 4$ oxidation states
Answer
520.2k+ views
Hint:Oxidation state is defined as the total number of electrons lost or gained in order to form a chemical bond with another atom. In sodium thiosulphate two sulphur atoms are present. The two types of sulphur are present in different forms i.e. coordinate bonds between the acceptor sulphur atom and central sulphur atom due to which their oxidation states are different which needs to be found out.
Complete answer:
We have been given sodium thiosulphate having the chemical formula $N{a_2}{S_2}{O_3}$ . We draw the structure of sodium thiosulphate
The structure is as follows
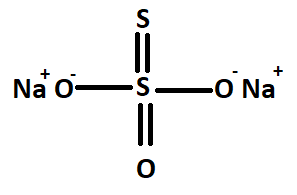
Here we can see that there are two types of a sulphur atom such as acceptor sulphur atom in which there is a coordinate bond between sulphur. So the oxidation state of acceptor sulphur atom is $ - 2$.
And we have to find the oxidation state of another sulphur atom.
Let the oxidation state of the central sulphur atom in sodium thiosulphate be x
Then we know the sum of all oxidation numbers of constituent atoms is equal to zero. Then we get the following
$2( + 1) + 3( - 2) + x + 1( - 2) = 0$
$ \Rightarrow x = + 6$
Here in the equation, the oxidation state of coordinate bond acceptor sulphur atom is $ - 2$
Therefore the oxidation state of sulphur atoms in $N{a_2}{S_2}{O_3}$ is $ - 2$ and $ + 6$.
Hence the correct answer is option C.
Note:
When the coordinate bond is formed between two same electronegative elements then the electron donor element shows $ + 2$ oxidation state. The electron donor element shows $ - 2$ oxidation state. Also, the coordinate bond carries two electrons so the acceptor sulphur atom has a charge of $ - 2$.
Complete answer:
We have been given sodium thiosulphate having the chemical formula $N{a_2}{S_2}{O_3}$ . We draw the structure of sodium thiosulphate
The structure is as follows

Here we can see that there are two types of a sulphur atom such as acceptor sulphur atom in which there is a coordinate bond between sulphur. So the oxidation state of acceptor sulphur atom is $ - 2$.
And we have to find the oxidation state of another sulphur atom.
Let the oxidation state of the central sulphur atom in sodium thiosulphate be x
Then we know the sum of all oxidation numbers of constituent atoms is equal to zero. Then we get the following
$2( + 1) + 3( - 2) + x + 1( - 2) = 0$
$ \Rightarrow x = + 6$
Here in the equation, the oxidation state of coordinate bond acceptor sulphur atom is $ - 2$
Therefore the oxidation state of sulphur atoms in $N{a_2}{S_2}{O_3}$ is $ - 2$ and $ + 6$.
Hence the correct answer is option C.
Note:
When the coordinate bond is formed between two same electronegative elements then the electron donor element shows $ + 2$ oxidation state. The electron donor element shows $ - 2$ oxidation state. Also, the coordinate bond carries two electrons so the acceptor sulphur atom has a charge of $ - 2$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




