
The total number of stereoisomers of \[{\rm{2,3 - }}\] dibromobutane is ?
(A) 2
(B) 3
(C) 4
(D) 5
Answer
591.3k+ views
Hint: As we know that the structure of \[{\rm{2,3 - }}\] dibromobutane is shown as \[C{H_3} - CHBr - CHBr - C{H_3}\]. The non- superimposable mirror images of \[{\rm{2,3 - }}\] dibromobutane will give the stereoisomers.
Complete step by step answer:
Stereoisomerism-The isomers in which the structures of compounds are same but the spatial arrangements of substituents are different are called stereoisomers.
The stereoisomers are of two types;
Geometrical isomers - The molecules which have cis and trans geometry.
Optical isomers - If the molecule does not contain any plane of symmetry and also the molecule does not give superimposable mirror images then it is known as optical isomers.
If the bromine atom is present above the plane as shown in figure-
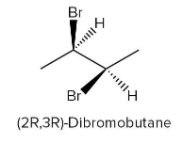
If the bromine atom is present below the plane as shown in figure-
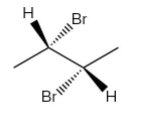

Both of These are enantiomers.
If the one bromine atom is present above the plane and one below the plane as shown in figure-
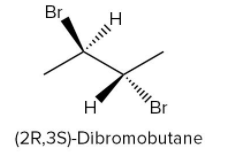
It also gives a stereoisomerism of \[{\rm{2,3 - }}\] dibromobutane.
Therefore, the total number of stereoisomers of \[{\rm{2,3 - }}\]dibromobutane is three.
The correct option is (B).
Note: When the molecule contains four different groups or atoms then it is known as chiral molecule. The chiral molecule rotates the plane polarised light in either left or right direction to the direction of plane polarization then the molecule is known as optically active.
The optically active molecule gives the pair of enantiomers which are non-superimposable mirror images.
Complete step by step answer:
Stereoisomerism-The isomers in which the structures of compounds are same but the spatial arrangements of substituents are different are called stereoisomers.
The stereoisomers are of two types;
Geometrical isomers - The molecules which have cis and trans geometry.
Optical isomers - If the molecule does not contain any plane of symmetry and also the molecule does not give superimposable mirror images then it is known as optical isomers.
If the bromine atom is present above the plane as shown in figure-

If the bromine atom is present below the plane as shown in figure-

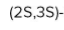

Both of These are enantiomers.
If the one bromine atom is present above the plane and one below the plane as shown in figure-

It also gives a stereoisomerism of \[{\rm{2,3 - }}\] dibromobutane.
Therefore, the total number of stereoisomers of \[{\rm{2,3 - }}\]dibromobutane is three.
The correct option is (B).
Note: When the molecule contains four different groups or atoms then it is known as chiral molecule. The chiral molecule rotates the plane polarised light in either left or right direction to the direction of plane polarization then the molecule is known as optically active.
The optically active molecule gives the pair of enantiomers which are non-superimposable mirror images.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




