
The total number of sigma $ \sigma $ and pi $ \pi $ bonds present in $ {C_2}{\left( {CN} \right)_4} $ are:
(A) $ 9\sigma $ and $ 9\pi $
(B) $ 9\sigma $ and $ 18\pi $
(C) $ 18\sigma $ and $ 9\pi $
(D) $ 18\sigma $ and $ 18\pi $
Answer
515.4k+ views
Hint: Sigma and pi bonds are nothing but just the types of covalent bonds. They differ in the overlapping of atomic orbitals. When there is a head-to-head overlapping of atomic orbitals, sigma bonds are formed whereas when there is a lateral overlapping of two atomic orbitals, formation of pi bonds takes place.
Complete answer:
There are $ 9\sigma $ and $ 9\pi $ bonds present in $ {C_2}{\left( {CN} \right)_4} $ . Therefore option A is the correct option. The structure of the compound is given below.
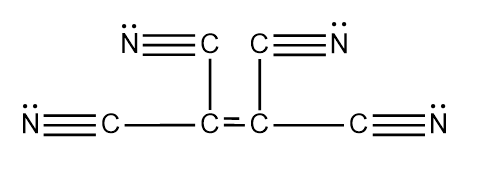
In a triple bond there are two pi bonds and one sigma bond is present. As there are four triple bonds, therefore, there are four sigma bonds and eight pi bonds are present. Now, there is also the presence of a single bond which is also called the sigma bond. So therefore, there are a total of four single bonds which means that there are four sigma bonds. Now one double bond is also present in it. In a double bond, there is one sigma bond and one pi bond. So when we total them together we find that there are a total of nine sigma bonds and nine pi bonds $ \left( {9\sigma {\text{ and 9}}\pi } \right) $ .
Note:
In triple bonds two pi bonds are present, one at the upper side and the other at the lower side. Sigma bond is present at the middle of the bond. In a double bond, one sigma and one pi bond is present. Pi bond is at the upper side and sigma bond is at the lower side of the bond.
Complete answer:
There are $ 9\sigma $ and $ 9\pi $ bonds present in $ {C_2}{\left( {CN} \right)_4} $ . Therefore option A is the correct option. The structure of the compound is given below.

In a triple bond there are two pi bonds and one sigma bond is present. As there are four triple bonds, therefore, there are four sigma bonds and eight pi bonds are present. Now, there is also the presence of a single bond which is also called the sigma bond. So therefore, there are a total of four single bonds which means that there are four sigma bonds. Now one double bond is also present in it. In a double bond, there is one sigma bond and one pi bond. So when we total them together we find that there are a total of nine sigma bonds and nine pi bonds $ \left( {9\sigma {\text{ and 9}}\pi } \right) $ .
Note:
In triple bonds two pi bonds are present, one at the upper side and the other at the lower side. Sigma bond is present at the middle of the bond. In a double bond, one sigma and one pi bond is present. Pi bond is at the upper side and sigma bond is at the lower side of the bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




