
The total number of sigma and pi bonds in the following molecule?
(a)- ${{C}_{2}}{{H}_{2}}$
(b)- ${{C}_{2}}{{H}_{4}}$
Answer
590.7k+ views
Hint: If the compound is having all single bonds then all the bonds are sigma bonds. If the compounds have a double bond then 1 bond is a sigma bond and 1 bond is a pi bond. If the compounds have a triple bond then there are 2 pi bonds and 1 sigma bond.
Complete step by step answer:
When a molecule is formed by the covalent bond, 2 types of bonds are formed between the molecules. These are sigma bonds represented as $\sigma -bond$ and pi bond represented as $\pi -bond$.
When a bond is formed between two atoms by the overlap of their atomic orbitals along the intermolecular axis (end to end or head to head on the overlap), the bond formed is called a sigma ($\sigma $) bond.
Pi ($\pi $) bond is formed by lateral or sideways overlapping of p-orbitals, i.e., by overlapping of p-orbital in a direction at right angles to the internuclear axis.
(a)- ${{C}_{2}}{{H}_{2}}$
This compound is ethyne, a member of the alkyne group so it would have a triple bond between the carbon atoms.
Let us see the structure of ${{C}_{2}}{{H}_{2}}$
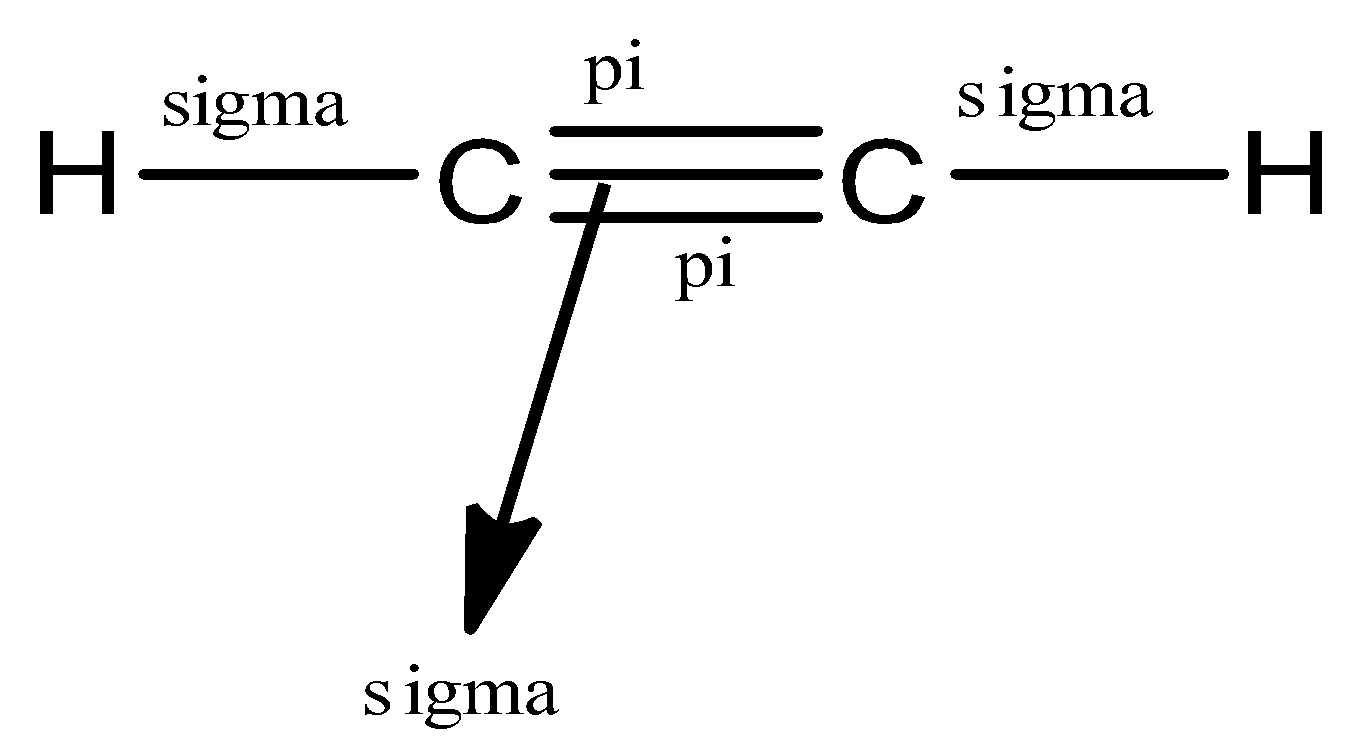
So, in ethyne, there are a total of 5 bonds, 3 sigma bonds, and 2 pi bonds.
(b)- ${{C}_{2}}{{H}_{4}}$
This compound is ethane and is a member of the alkene group. So, it would have a double bond between the carbon atoms.
Let us see the structure of ethane:
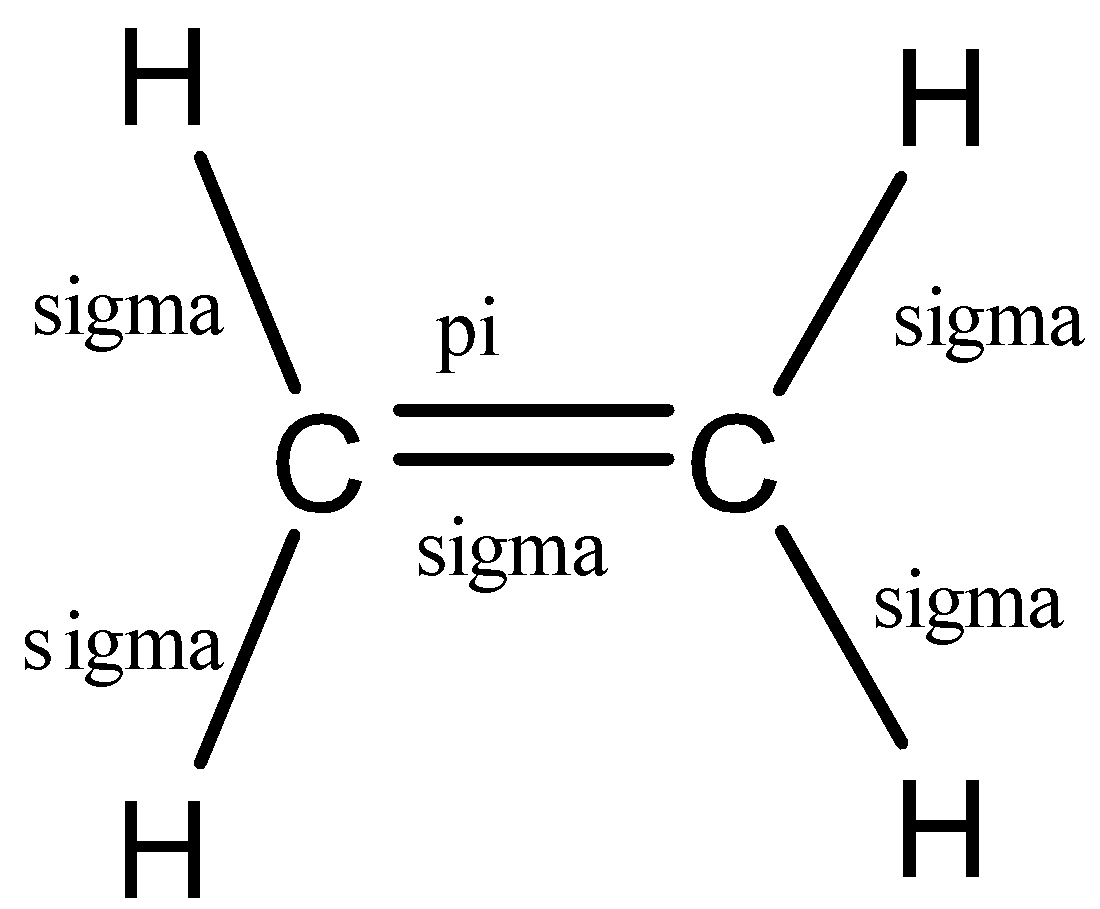
So, in ethane, there are 6 bonds, 5 sigma bonds, and 1 pi bond.
Note: The overlapping of the sigma bond is quite large and the sigma bond is strong. But the pi bond has a small extent of overlapping and it is a weak bond. The Sigma bond has only one electron cloud but the pi bond has a two-electron cloud, one above the plane of atomic nuclei and the other below it.
Complete step by step answer:
When a molecule is formed by the covalent bond, 2 types of bonds are formed between the molecules. These are sigma bonds represented as $\sigma -bond$ and pi bond represented as $\pi -bond$.
When a bond is formed between two atoms by the overlap of their atomic orbitals along the intermolecular axis (end to end or head to head on the overlap), the bond formed is called a sigma ($\sigma $) bond.
Pi ($\pi $) bond is formed by lateral or sideways overlapping of p-orbitals, i.e., by overlapping of p-orbital in a direction at right angles to the internuclear axis.
(a)- ${{C}_{2}}{{H}_{2}}$
This compound is ethyne, a member of the alkyne group so it would have a triple bond between the carbon atoms.
Let us see the structure of ${{C}_{2}}{{H}_{2}}$

So, in ethyne, there are a total of 5 bonds, 3 sigma bonds, and 2 pi bonds.
(b)- ${{C}_{2}}{{H}_{4}}$
This compound is ethane and is a member of the alkene group. So, it would have a double bond between the carbon atoms.
Let us see the structure of ethane:

So, in ethane, there are 6 bonds, 5 sigma bonds, and 1 pi bond.
Note: The overlapping of the sigma bond is quite large and the sigma bond is strong. But the pi bond has a small extent of overlapping and it is a weak bond. The Sigma bond has only one electron cloud but the pi bond has a two-electron cloud, one above the plane of atomic nuclei and the other below it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




