
The total number of lone pair of electrons in the given molecule is:
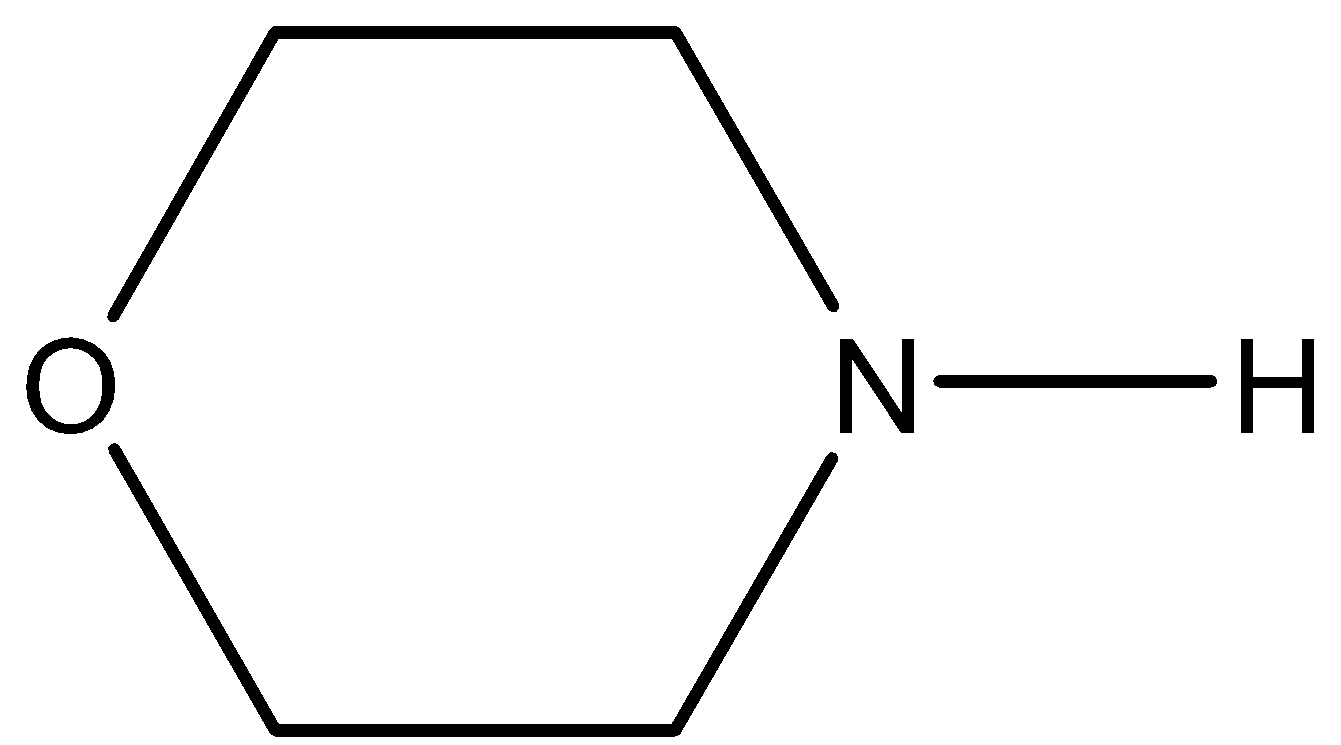
A. 2
B. 3
C. 4
D. 5

Answer
599.1k+ views
Hint: In chemistry, a lone pair of electrons means a pair of valence electrons that are not shared with another atom at the formation of a covalent bond and is occasionally called an unshared pair or non-bonding pair.
Complete step by step answer:
- Lone pairs are found in the outermost electron shell of atoms.
- Lone pair of electrons can be identified by using a Lewis structure.
- The Lewis structure of the given molecule is as follows.
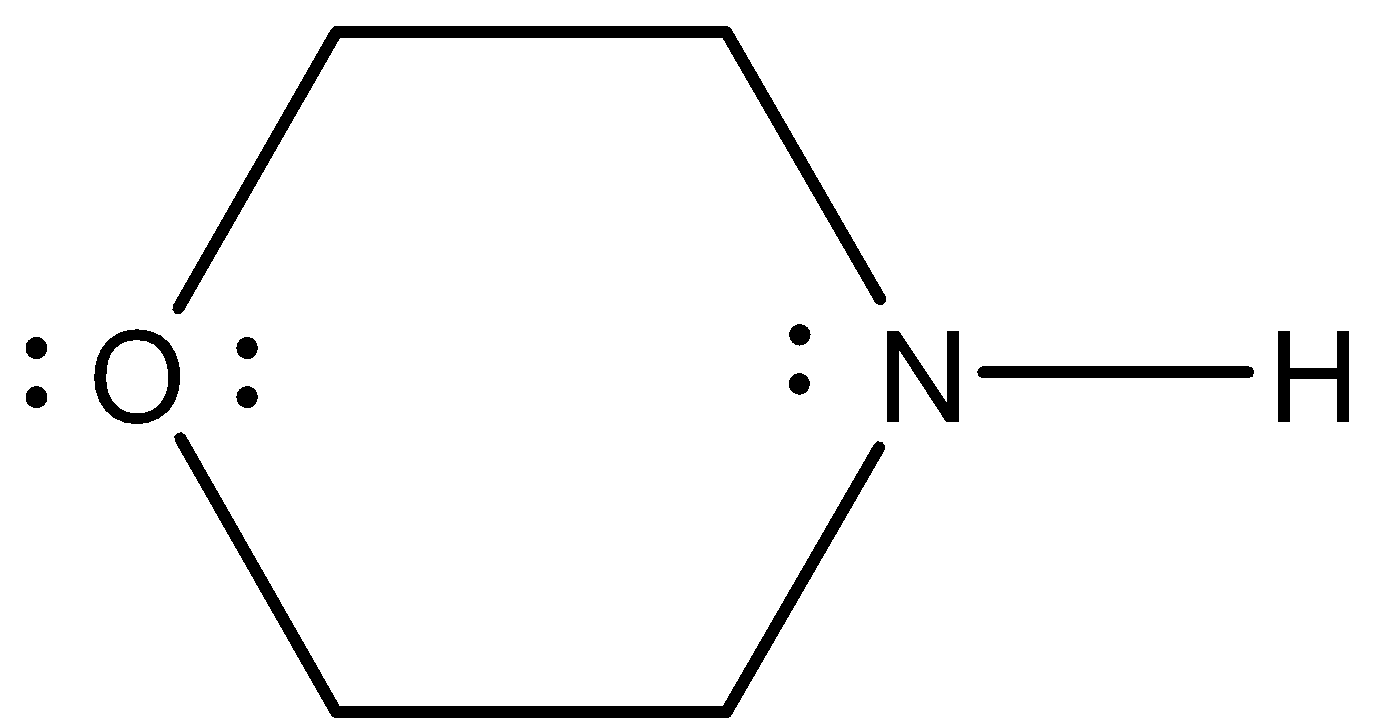
- In Lewis structure the lone pair of electrons denoted on the respective atoms is very clear. So, we can count them very easily by using Lewis structure.
- Now, coming to given options, option A, 2. It is wrong because the given molecule contains more than two lone pairs of electrons in its structure.
- Coming to option C, 4. It is wrong because the given molecule contains less than four lone pairs of electrons in its structure.
- Coming to option D, 5. It is wrong because the given molecule contains less than four lone pairs of electrons in its structure.
- Coming to option C, 3. Yes the given molecule contains three lone pairs of electrons. Two lone pairs on oxygen atoms and one lone pair on nitrogen atoms.
So, the correct option is C.
Note: Don’t be confused with the terms non-bonding electrons and lone pair of electrons. Both are the same.
Lone pair of electrons: the electrons which do not involve in bonding with other atoms are called lone pairs of electrons.
Complete step by step answer:
- Lone pairs are found in the outermost electron shell of atoms.
- Lone pair of electrons can be identified by using a Lewis structure.
- The Lewis structure of the given molecule is as follows.

- In Lewis structure the lone pair of electrons denoted on the respective atoms is very clear. So, we can count them very easily by using Lewis structure.
- Now, coming to given options, option A, 2. It is wrong because the given molecule contains more than two lone pairs of electrons in its structure.
- Coming to option C, 4. It is wrong because the given molecule contains less than four lone pairs of electrons in its structure.
- Coming to option D, 5. It is wrong because the given molecule contains less than four lone pairs of electrons in its structure.
- Coming to option C, 3. Yes the given molecule contains three lone pairs of electrons. Two lone pairs on oxygen atoms and one lone pair on nitrogen atoms.
So, the correct option is C.
Note: Don’t be confused with the terms non-bonding electrons and lone pair of electrons. Both are the same.
Lone pair of electrons: the electrons which do not involve in bonding with other atoms are called lone pairs of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




