
The third line of the Balmer series in the emission spectrum of the hydrogen atom is due to the transition from the?
a.) Forth bohr orbit to the first bohr orbit
b.) Fifth bohr orbit to the secondary bohr orbit
c.) Sixth bohr orbit to the third bohr orbit
d.) Seventh bohr orbit to the third bohr orbit
Answer
599.1k+ views
Hint: Balmer lines appear in numerous stellar objects due to the abundance of hydrogen in the universe. The longest wavelength in the balmer series is 656. The balmer series is atomic hydrogen. The wavelength of these lines are :$1/\lambda = {R_H}(1/4 - 1/{n^2})$, where $\lambda $ is a wavelength, ${R_H}$ is Rydberg constant, and n is the original orbit level.
Complete answer:
In this given question the term emission spectrum means an electron which goes from high orbit to low orbit. As we know that in balmer lines this is fixed to (n=2) as you can see in the figure given below. According to the question the first option shows the lyman series not the balmer series. In the Lyman series we talk about the first orbit therefore, the wavelength of the first line of the Lyman series in hydrogen atom is ‘1216’. The second option (b) is correct because in that we are talking about the balmer series. Moreover, the option(c, d) we are talking about is the paschen series which means (n=3). Whereas the shortest wavelength of paschen series is ${10^7}{m^{ - 1}}$ and the longest wavelength of paschen series is $18752{A^o}$
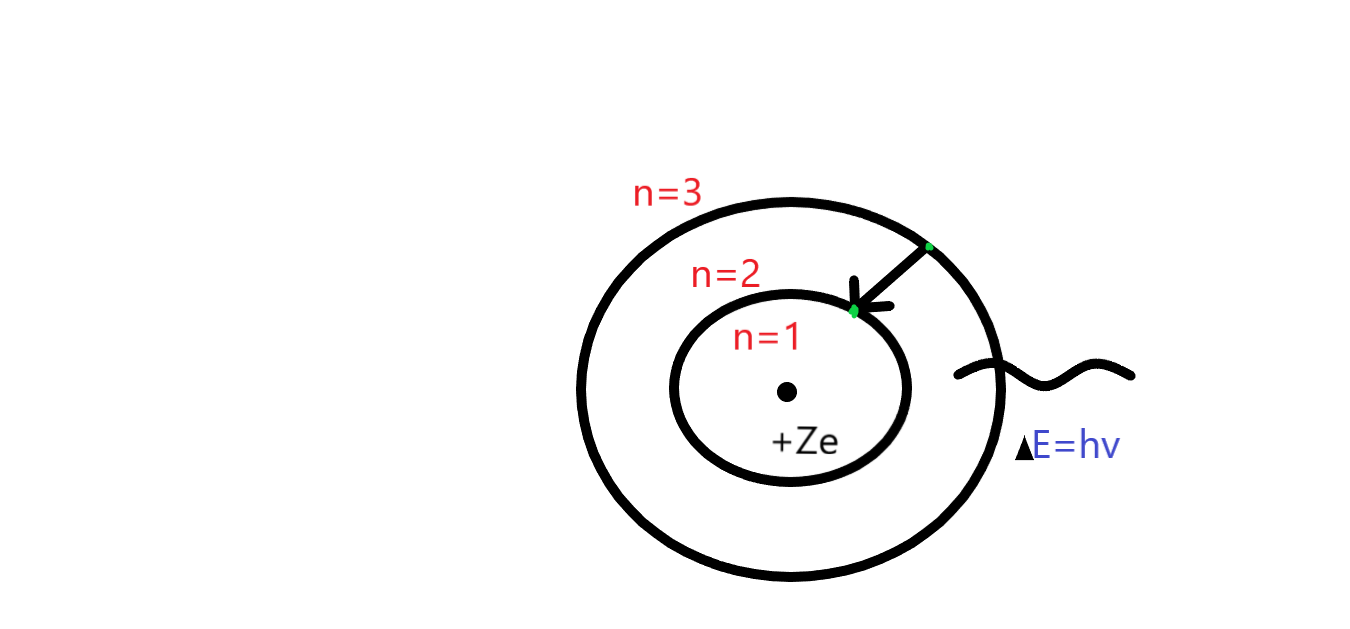
Note: In lyman series the main spectral lines are visible as ultra-violet, paschen, brackett and pfund all these series the main spectral lines are visible as infra-red. In Lyman series n=1, balmer series n=2, paschen series n=3, barckett series n=4, pfund series n=5.
Complete answer:
In this given question the term emission spectrum means an electron which goes from high orbit to low orbit. As we know that in balmer lines this is fixed to (n=2) as you can see in the figure given below. According to the question the first option shows the lyman series not the balmer series. In the Lyman series we talk about the first orbit therefore, the wavelength of the first line of the Lyman series in hydrogen atom is ‘1216’. The second option (b) is correct because in that we are talking about the balmer series. Moreover, the option(c, d) we are talking about is the paschen series which means (n=3). Whereas the shortest wavelength of paschen series is ${10^7}{m^{ - 1}}$ and the longest wavelength of paschen series is $18752{A^o}$

Note: In lyman series the main spectral lines are visible as ultra-violet, paschen, brackett and pfund all these series the main spectral lines are visible as infra-red. In Lyman series n=1, balmer series n=2, paschen series n=3, barckett series n=4, pfund series n=5.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




