
The thermometer in the diagram has no scale. Where must the bulb be placed so that $ {0^0}C $ can be marked on the stem?
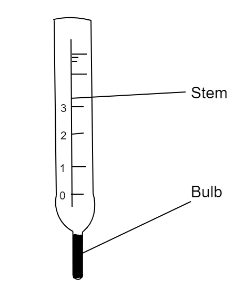
(A) In freezer
(B) In pure boiling water
(C) In pure cold water
(D) In pure melting ice

Answer
479.4k+ views
Hint: Freezer usually has a temperature of $ - {18^0}C $ . Water is considered to be boiling when the vapour pressure of water equals the atmospheric pressure, at sea level to make the vapour pressure and atmospheric pressure equal a temperature of $ {100^0}C $ is needed.
Complete Step By Step Answer:
Because ice melts at $ {0^0}C $ it will be the perfect choice for dipping the thermometer to ensure $ {0^0}C $ on the scale. Normally, at any temperature less than $ {0^0}C $ water will be present only in solid state and at $ {0^0}C $ both solid and liquid state coexist. For any rise from this temperature the ice will be melted and only liquid form will exist.
In a freezer the temperature can fall below zero degrees so it is not possible to measure $ {0^0}C $ using a thermometer without a scale.
In pure boiling water the temperature will be $ {100^0}C $ .
In pure cold water the temperature can be anywhere above $ {0^0}C $ so exact measurement is not possible.
Option (D) is the correct answer.
Note:
Water has a unique property called anomalous expansion of water. Due to this property when the temperature falls down from $ {4^0}C $ to $ {0^0}C $ its density increases and expands. So water is most dense when the temperature is $ {4^0}C $ . It behaves like any other substance until it reaches $ {4^0}C $ , this anomaly is only observed in case of water and no other liquid displays this property. Generally all other materials contract as temperature decreases and expands when temperature is raised.
Complete Step By Step Answer:
Because ice melts at $ {0^0}C $ it will be the perfect choice for dipping the thermometer to ensure $ {0^0}C $ on the scale. Normally, at any temperature less than $ {0^0}C $ water will be present only in solid state and at $ {0^0}C $ both solid and liquid state coexist. For any rise from this temperature the ice will be melted and only liquid form will exist.
In a freezer the temperature can fall below zero degrees so it is not possible to measure $ {0^0}C $ using a thermometer without a scale.
In pure boiling water the temperature will be $ {100^0}C $ .
In pure cold water the temperature can be anywhere above $ {0^0}C $ so exact measurement is not possible.
Option (D) is the correct answer.
Note:
Water has a unique property called anomalous expansion of water. Due to this property when the temperature falls down from $ {4^0}C $ to $ {0^0}C $ its density increases and expands. So water is most dense when the temperature is $ {4^0}C $ . It behaves like any other substance until it reaches $ {4^0}C $ , this anomaly is only observed in case of water and no other liquid displays this property. Generally all other materials contract as temperature decreases and expands when temperature is raised.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




