
The tetrahedral arrangement of perchlorate ion is due to:
(A) presence of a lone pair of electrons.
(B) trigonal bipyramidal shape of the ion.
(C) $ s{{p}^{3}} $ hybridisation.
(D) $ s{{p}^{2}} $ hybridisation.
Answer
527.4k+ views
Hint: We know that basically, chlorate ion has a molecular formula $ Cl{{O}^{3-}} $ . These are the salts of chloric acid. Further, the metal chlorates can be prepared by adding chlorine to metal hydroxides such as $ KOH $ . So, to solve this question we need to draw the structure and then determine its shape.
Complete answer:
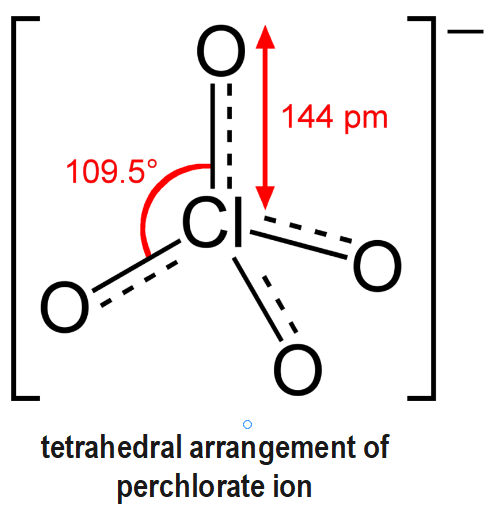
Chlorates are basically the salt of chloric acid. Some of the examples of chlorates are sodium chlorate, potassium chlorate, magnesium chlorate etc. Now, only one Lewis structure cannot represent the chlorate ion since all the bonds are of the same length and the chlorine atom is further hypervalent. So, it is considered as a hybrid of multiple resonance structures. In perchlorate ion $ O-Cl-O $ bond angle $ {{109.5}^{o}}~ $ due to four groups of bonding electrons and no lone pairs of electrons.
Due to $ s{{p}^{3}} $ hybridisation of $ Cl $ atom, it has a tetrahedral structure.
Now, the chlorate ion is $ s{{p}^{3}} $ hybridized with three bond pairs and one lone pair, so its shape is pyramidal. Moreover, three oxygen atoms will have one unpaired orbital and the chlorine’s unoccupied d orbitals will be used for bonding.
Therefore, correct answer is option C.
Note:
Remember that the chlorates are powerful oxidizers and should be kept away from easily oxidized materials. Moreover, the mixtures of chlorate salts with combustible material like charcoal, organic solvents, and metals will readily deflategate. These were widely used in pyrotechnics but not their use has fallen due to their instability. Further, these are relatively toxic, though they form generally harmless chlorides on reduction.
Complete answer:
Chlorates are basically the salt of chloric acid. Some of the examples of chlorates are sodium chlorate, potassium chlorate, magnesium chlorate etc. Now, only one Lewis structure cannot represent the chlorate ion since all the bonds are of the same length and the chlorine atom is further hypervalent. So, it is considered as a hybrid of multiple resonance structures. In perchlorate ion $ O-Cl-O $ bond angle $ {{109.5}^{o}}~ $ due to four groups of bonding electrons and no lone pairs of electrons.
Due to $ s{{p}^{3}} $ hybridisation of $ Cl $ atom, it has a tetrahedral structure.

Now, the chlorate ion is $ s{{p}^{3}} $ hybridized with three bond pairs and one lone pair, so its shape is pyramidal. Moreover, three oxygen atoms will have one unpaired orbital and the chlorine’s unoccupied d orbitals will be used for bonding.
Therefore, correct answer is option C.
Note:
Remember that the chlorates are powerful oxidizers and should be kept away from easily oxidized materials. Moreover, the mixtures of chlorate salts with combustible material like charcoal, organic solvents, and metals will readily deflategate. These were widely used in pyrotechnics but not their use has fallen due to their instability. Further, these are relatively toxic, though they form generally harmless chlorides on reduction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




